Overview of mRNA vaccine technology and what has been implemented in SMOBIO
SMOBIO Technology Inc. 2022-02-17
Introduction
The mRNA vaccine comes from a molecular engineering ensemble where RNA substance respective to target antigens is produced by in-vitro transcribing from a DNA vector built-in with its corresponding DNA sequence. The overall structure of messenger RNA (mRNA, from 5’to 3’ end) contains cap, 5’untranslated region (5’-UTR), open reading frame (ORF) of target antigen, 3’-UTR, and poly(A) tail. Owing to cell-free manufacturing, in-vitro transcribed RNA requires further processing to turn into applicable mRNA through capping and poly-adenylation. The principal of mRNA vaccine is different from traditional vaccine. With appropriate delivery technology entering into cell cytoplasm, target antigen based on mRNA code will be made by intrinsic translation machinery and then trigger immunity.
The advantage of mRNA vaccine relies on its fast development cycle and ease of gene synthesis, which enables to combat emerging and mutation-prone virus transmission such as SARS-CoV-2 pandemic. With the successful clinical experience in safety and efficacy of mRNA vaccines against COVID-19 by Pfizer/BioNTech and Moderna, mRNA technology has drawn world-wide attention and shown more promising in its development. Compared to other types of vaccine, development of mRNA vaccines needs more efforts on building its delivery system, preservation, and storage condition as well. The key technical workflows of mRNA vaccine are listed in following sections.

Illustration of mRNA vaccine production: from DNA manufacturing, mRNA production, to LNP package and then cold-chain storage
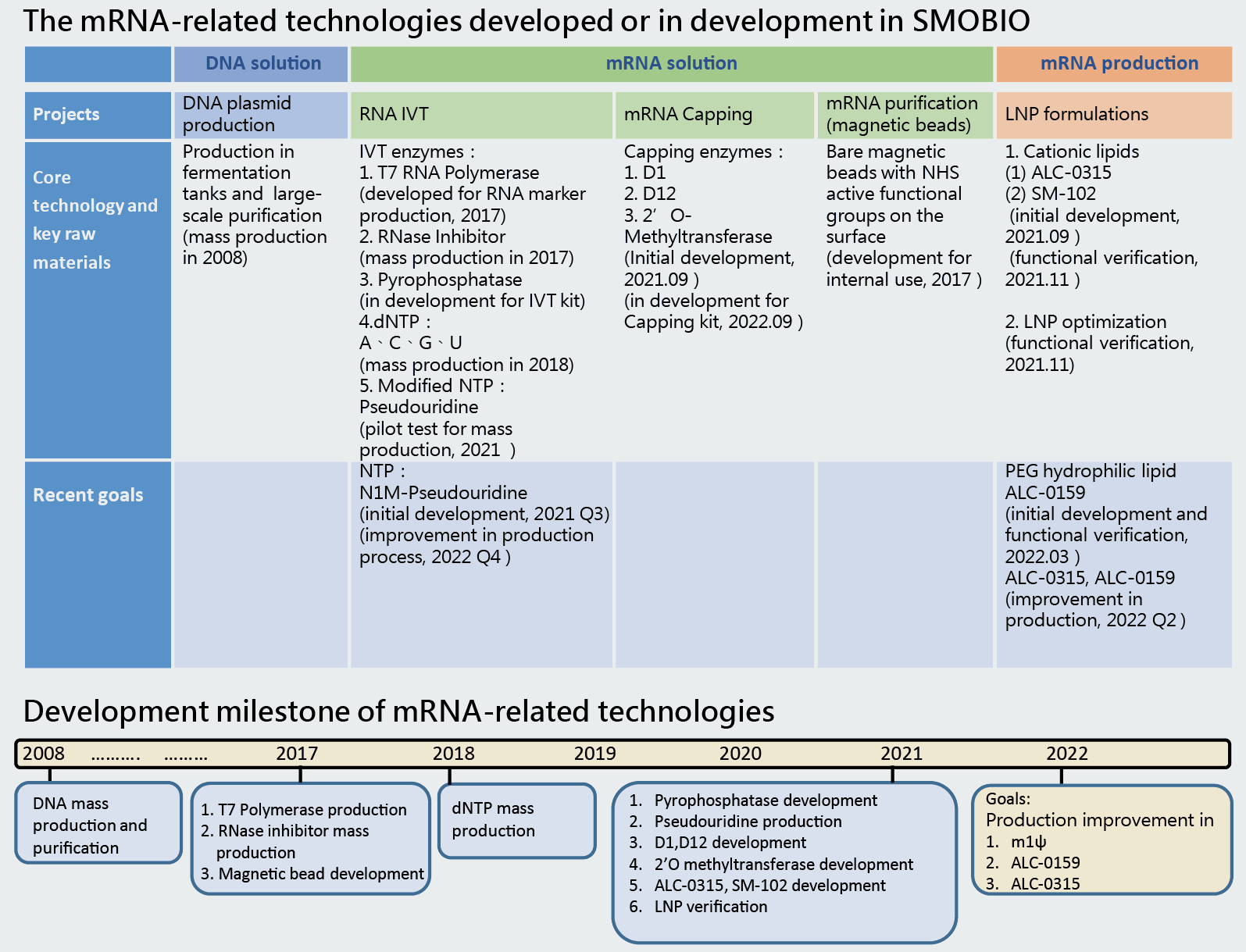
Technical highlight of SMOBIO in mRNA vaccine production
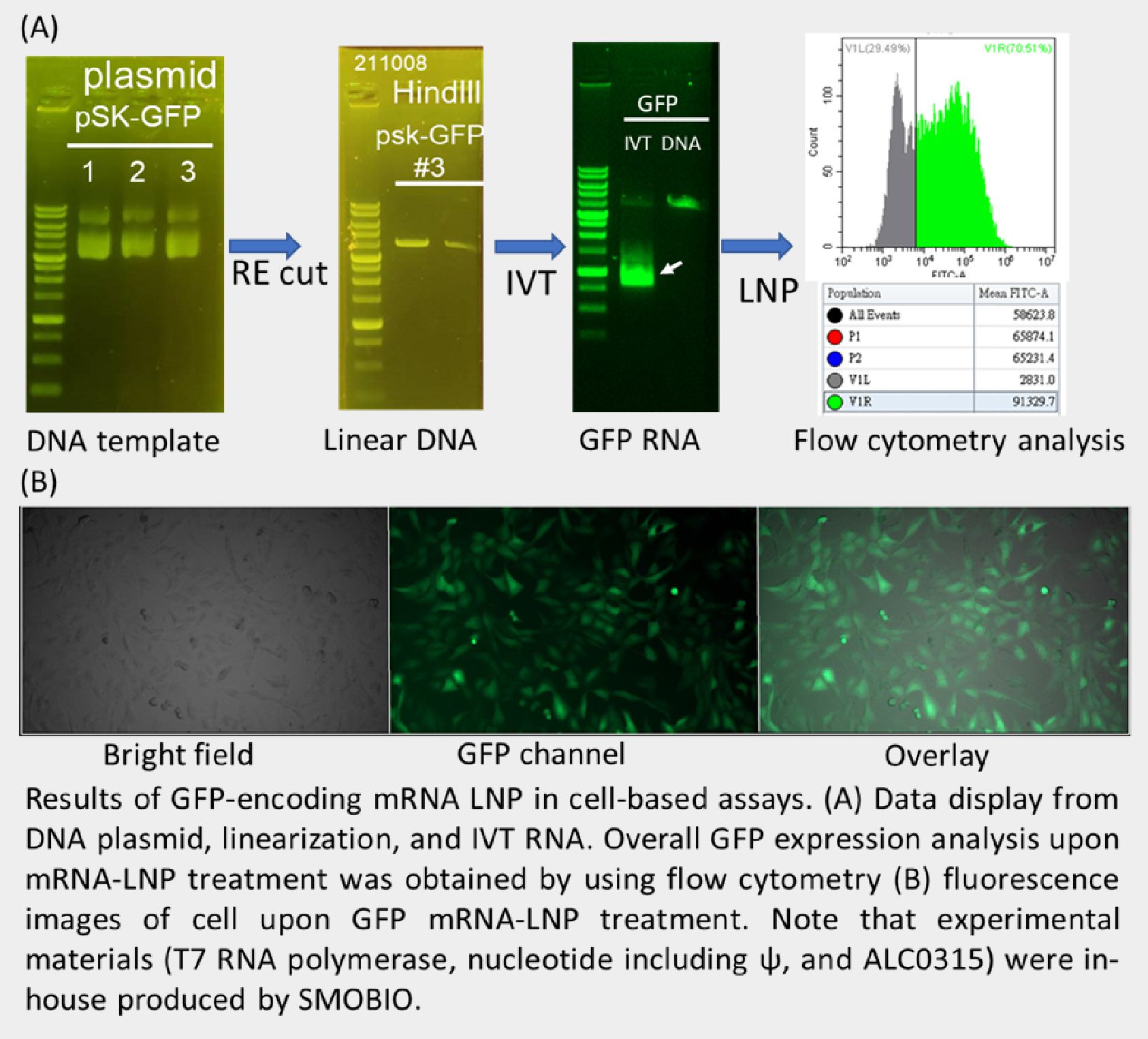
We have made mRNA-encoded eGFP and encapsulated it with LNP, then conducted functional test of mRNA-LNP in vitro. From cell based assay, it clearly displayed green fluorescent signal in living cells and broad expression efficiency by flow cytometry assessment (shown in following figures). This demonstrated that SMOBIO has ability and capability of mRNA-LNP manufacturing. Over past 2-year COVID pandemic, we hope to contribute our efforts on mRNA vaccine-related fields in Taiwan and world wide.
DNA plasmid manufacturing
1
.
DNA plasmid manufacturing
1.1 Plasmid DNA mutagenesis and construction in response to rapid virus evolution
1.2 Plasmid DNA mass production, including:
(1) Endotoxin removal
(2) RNA removal
(3) Genomic DNA removal
(1) Endotoxin removal
(2) RNA removal
(3) Genomic DNA removal
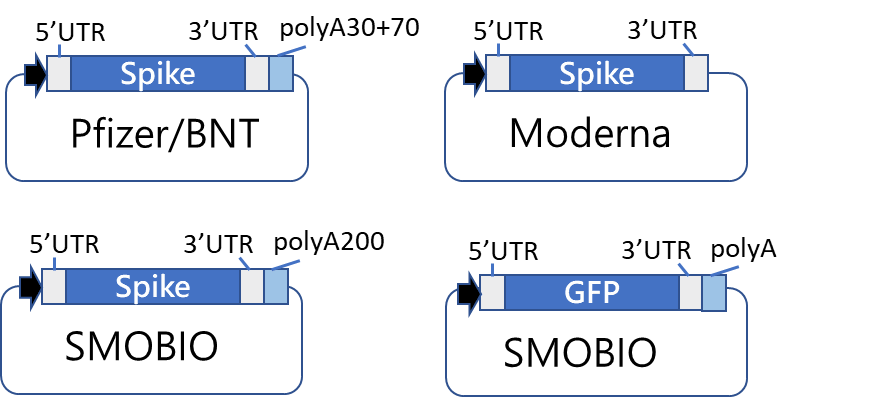
1.3 Current available DNA constructs
(1) Pfizer/BNT CoV-2 spike protein prefusion form, full length (Including Delta, C2, Mu derived mutants) (2) Moderna CoV-2 spike protein prefusion form, full length
(3) SMOBIO in-house designed CoV-2 spike protein prefusion form, full length(With longer poly(A) tails )
(4) EGFP reporter construct
1.4 Schematic view of available DNA template system (built with original DNA coding sequence)
Sequence Map Download:
pSK-BNT-Swt (based on BNT designed spike coding sequence and backbone) (Download)
pSK-Moderna-Swt (based on Moderna designed spike coding sequence) (Download)
pSK-SMBiO-Swt (SMOBIO in-house cassette design) (Download)
pSK-GFP (containing EGFP coding sequence) (Download)
2.
mRNA synthesis
2.1 In vitro transcription:
(1) T7 RNA polymerase
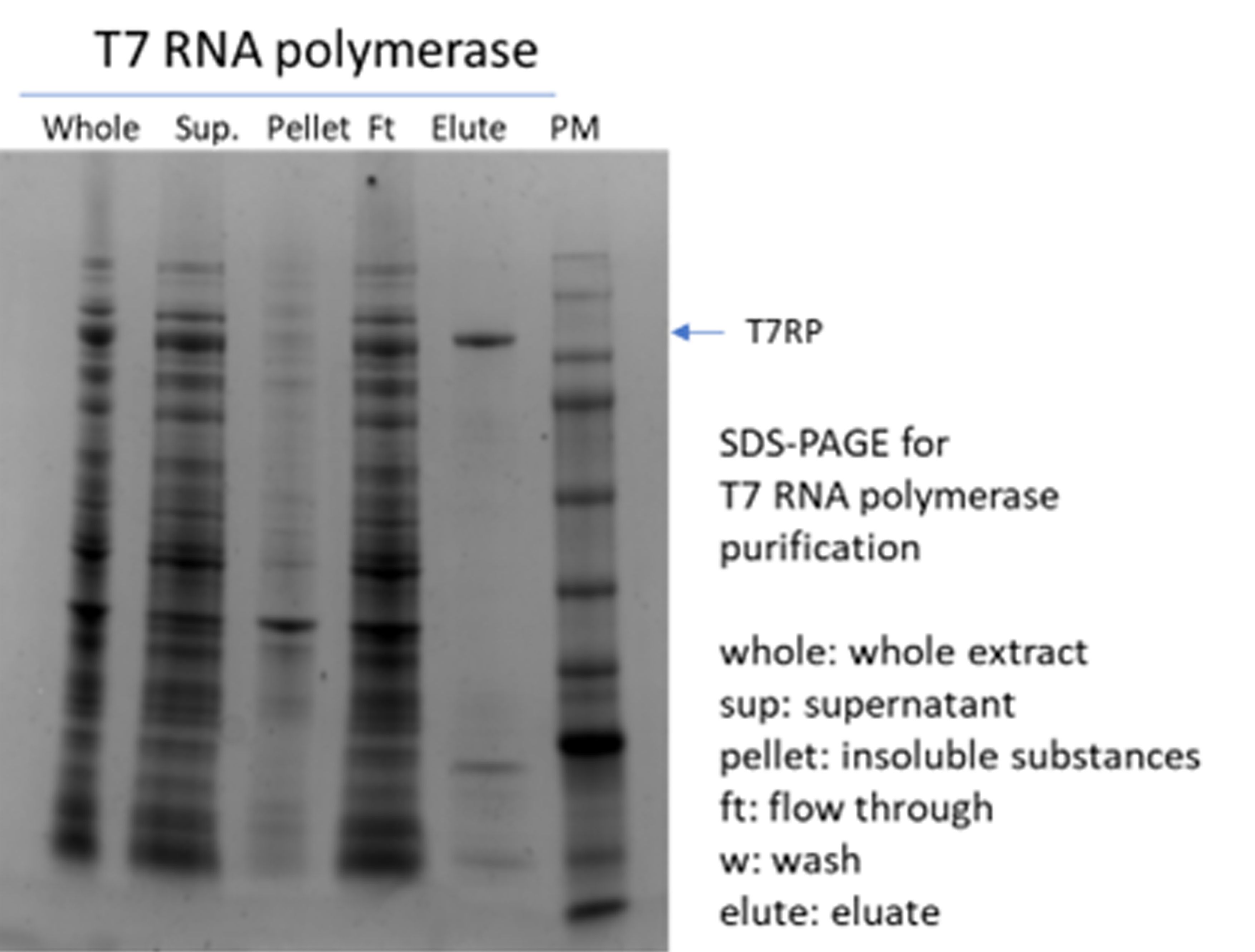
(2) RNase inhibitor
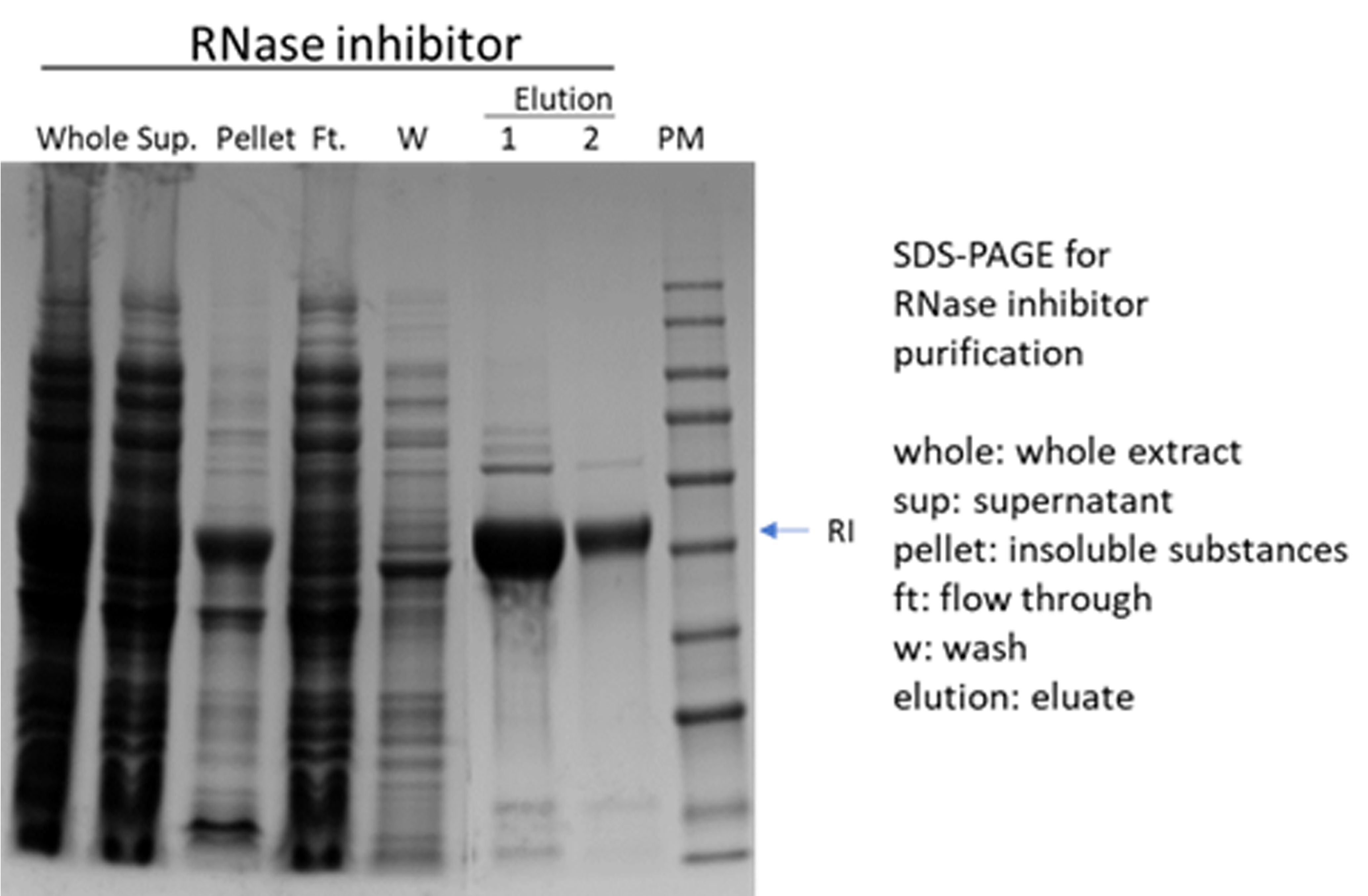
(3)
mRNA nucleotide building blocks:
NTP: ATP, UTP, CTP, GTP, ψTP, N1-methyl ψTP Nucleoside: ribose of A, U, C, G, ψ, N1-methyl ψ.
NTP: ATP, UTP, CTP, GTP, ψTP, N1-methyl ψTP Nucleoside: ribose of A, U, C, G, ψ, N1-methyl ψ.
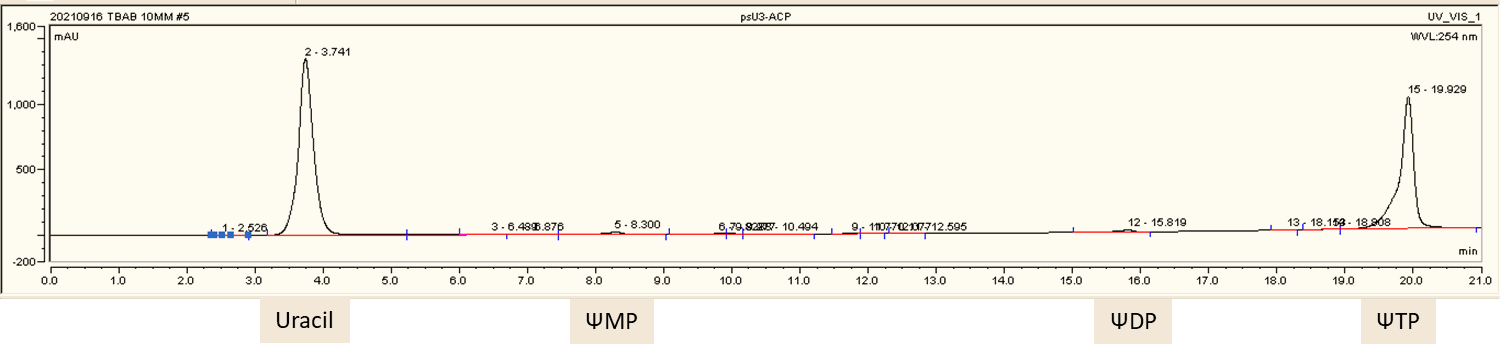
HPLC real-time monitoring for ψTP production
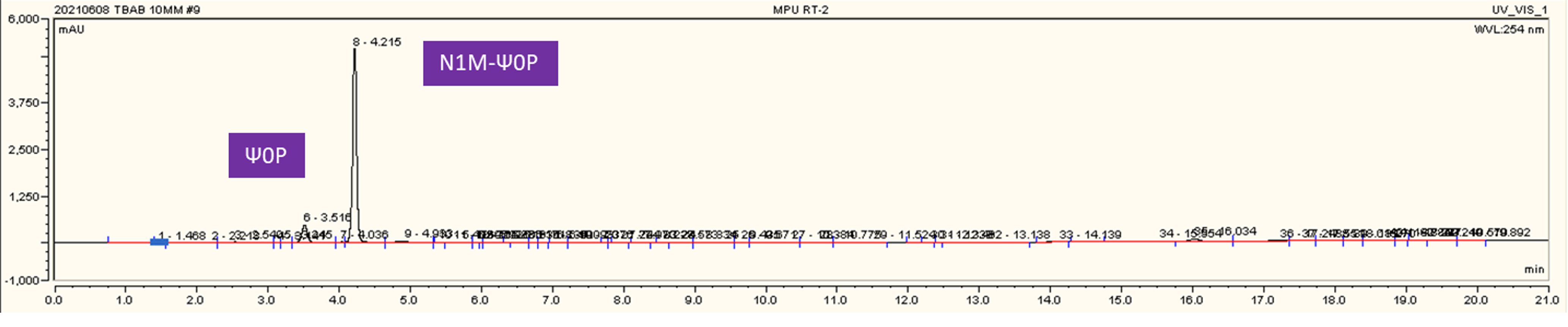
HPLC real-time monitoring for N1mψ0P production
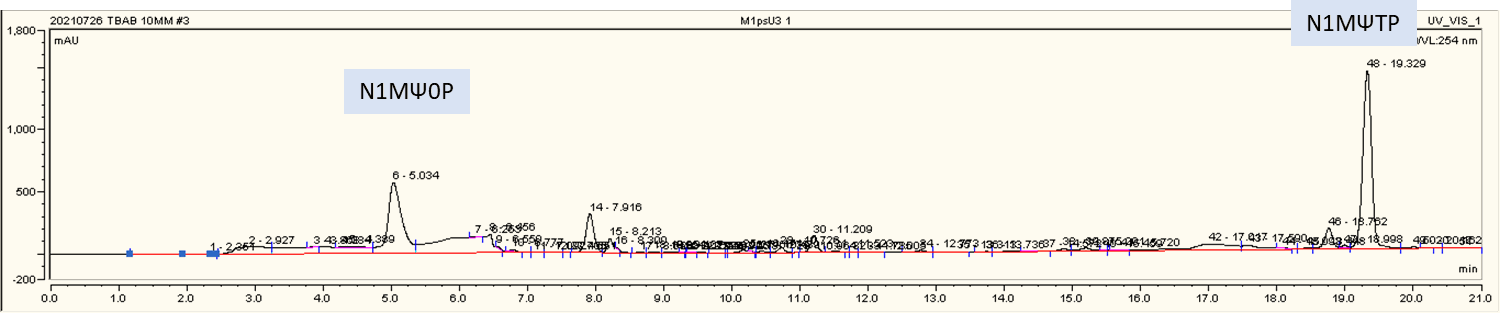
HPLC real-time monitoring for N1mψ0P production
Mass, NMR data are listed below:
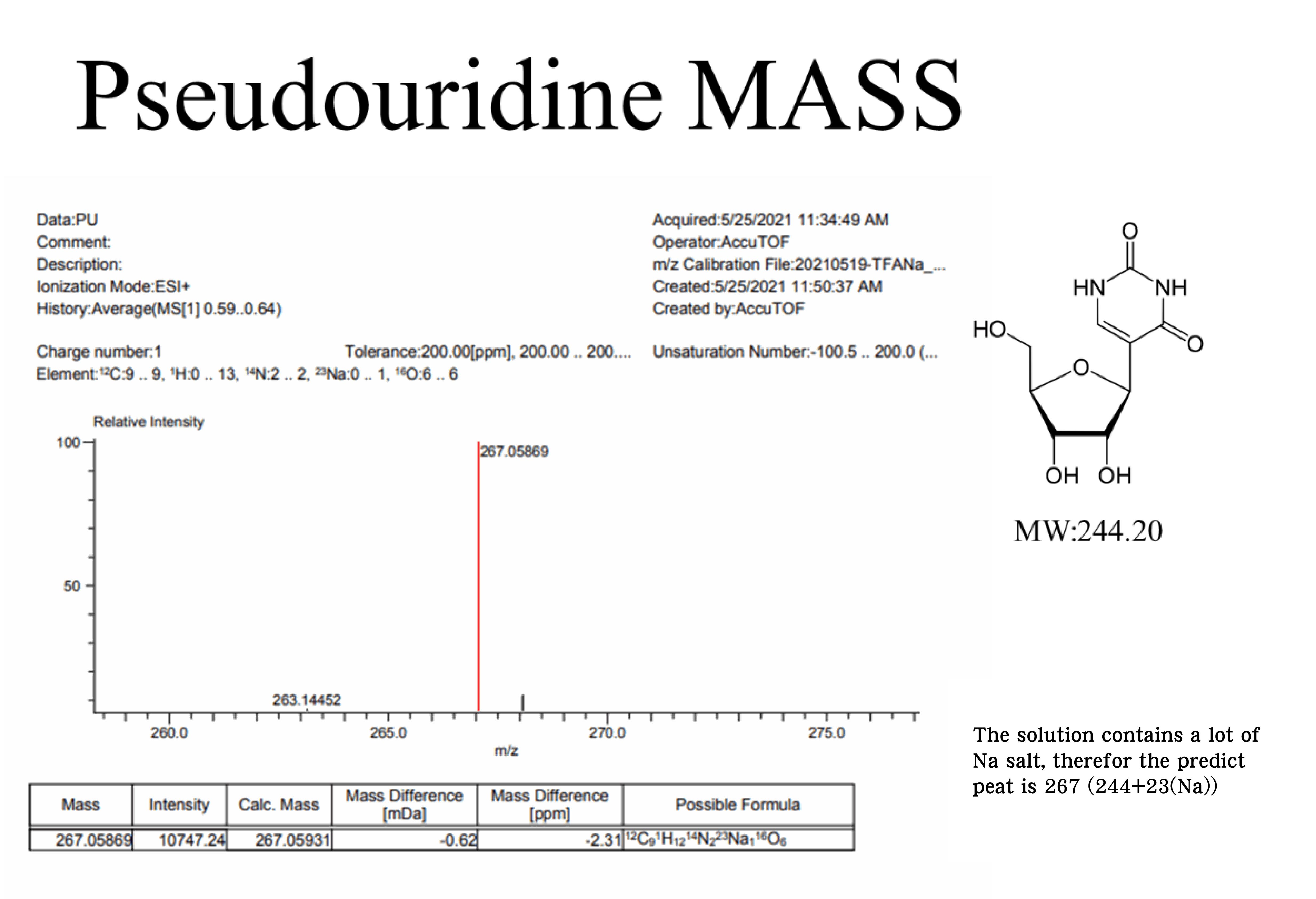
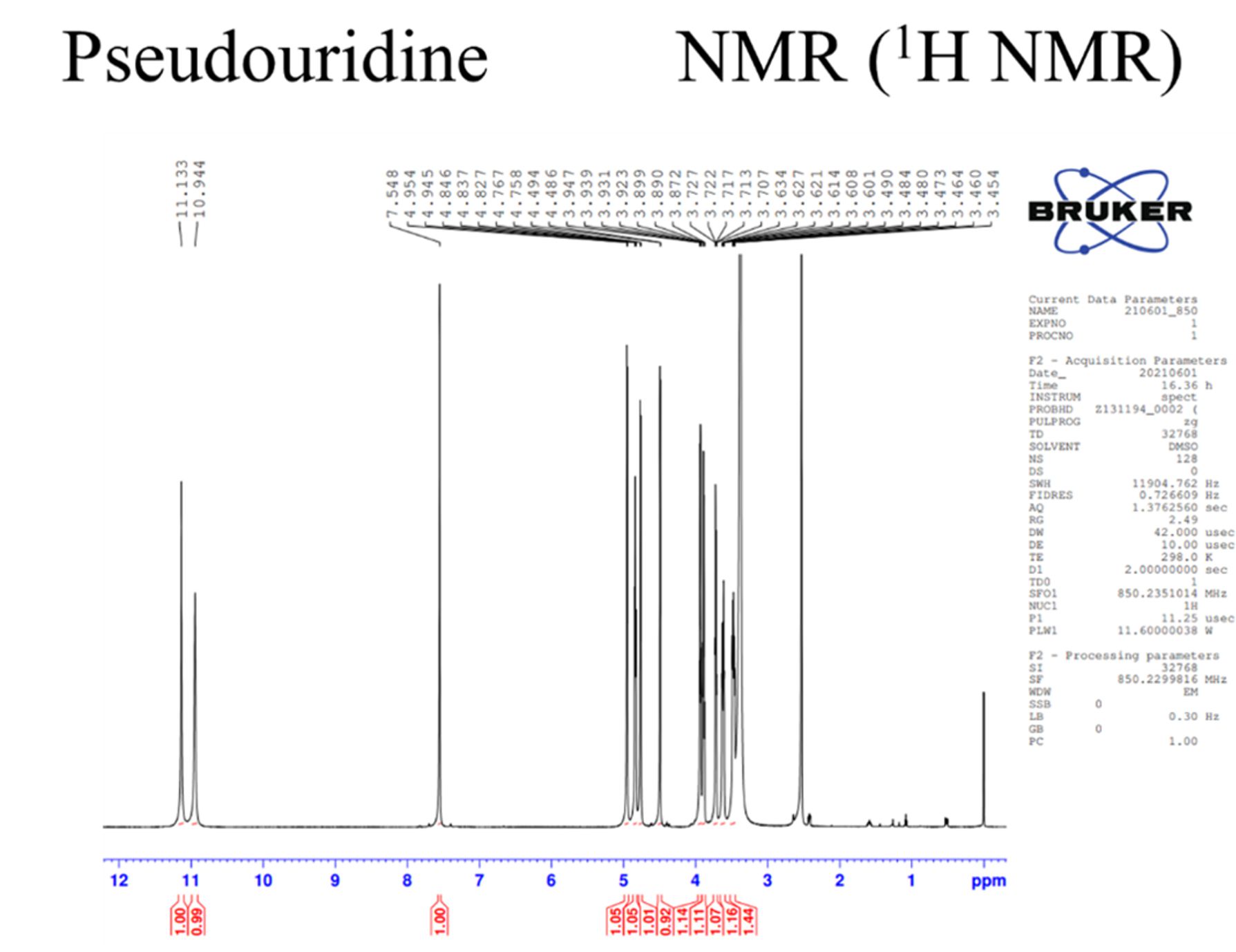
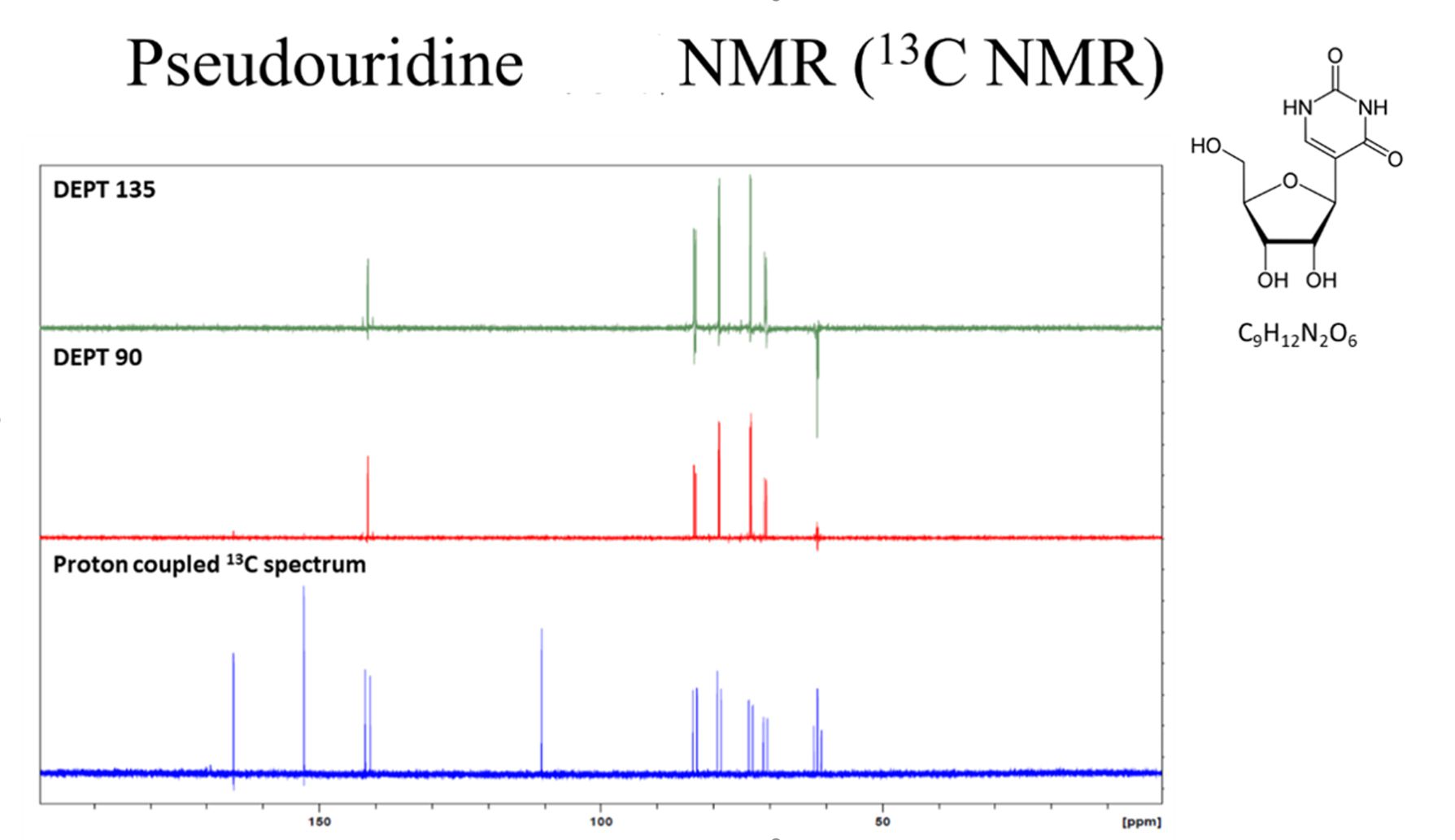
(4) Vaccinia caping enzyme: including D1, D12 subunit
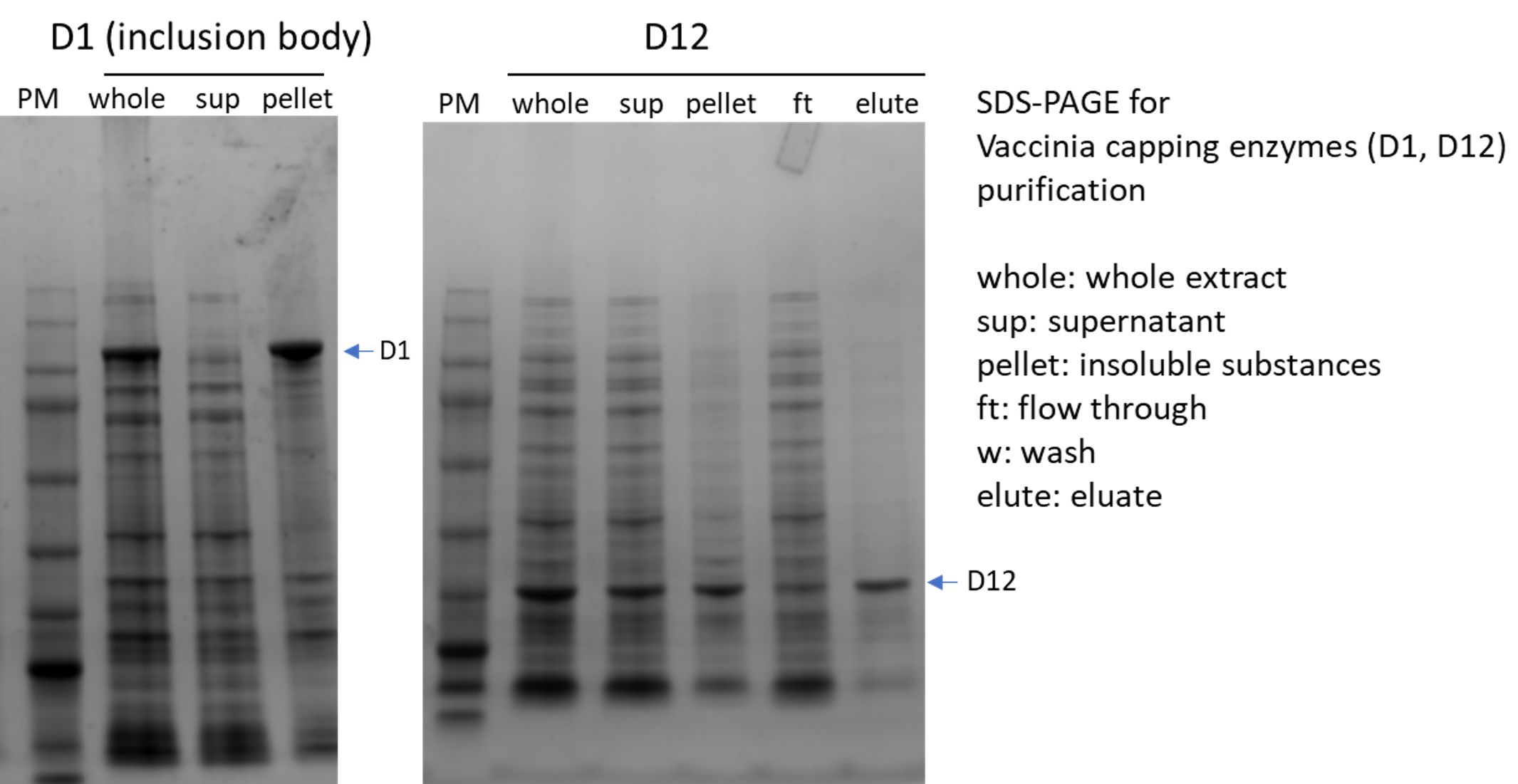
(5)2′-O-methyltransferase
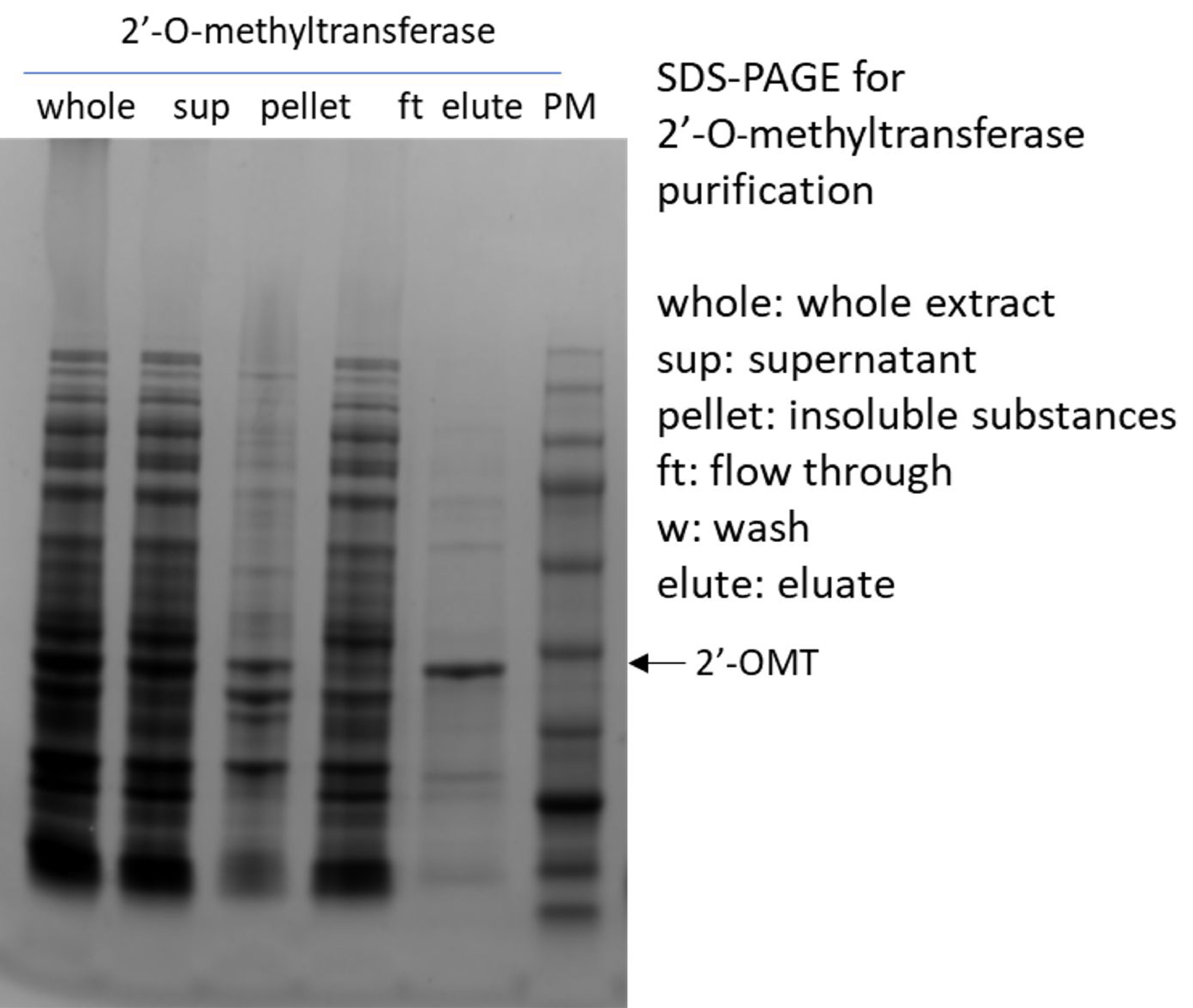
(6)Inorganic Pyrophosphatase
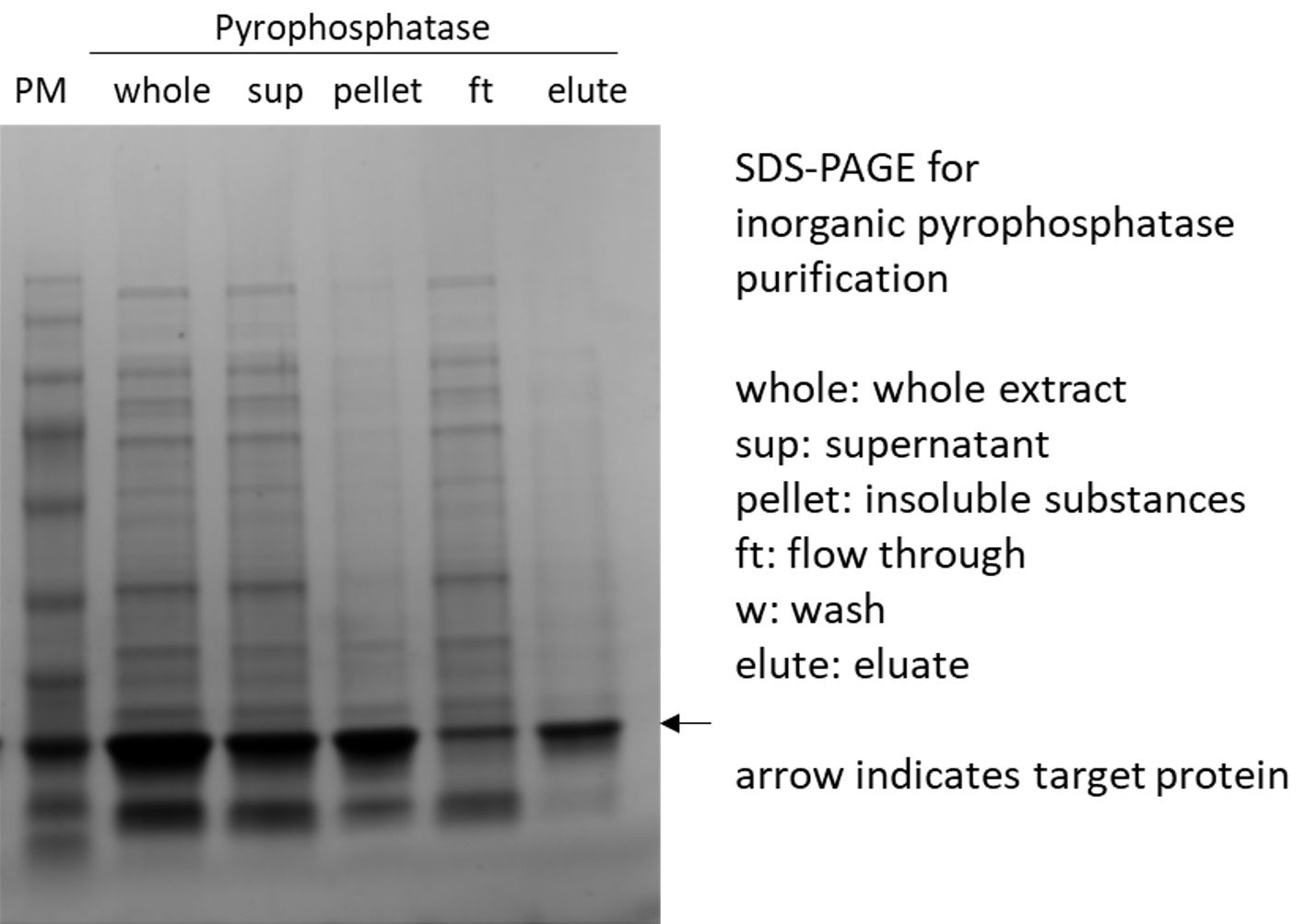
2.2 mRNA extraction: oligo-dT resin: developing in progress
3.
mRNA-LNP manufacturing
(1) key data about cationic lipid production
ALC 0315 cationic lipid production
ALC 0315 cationic lipid production
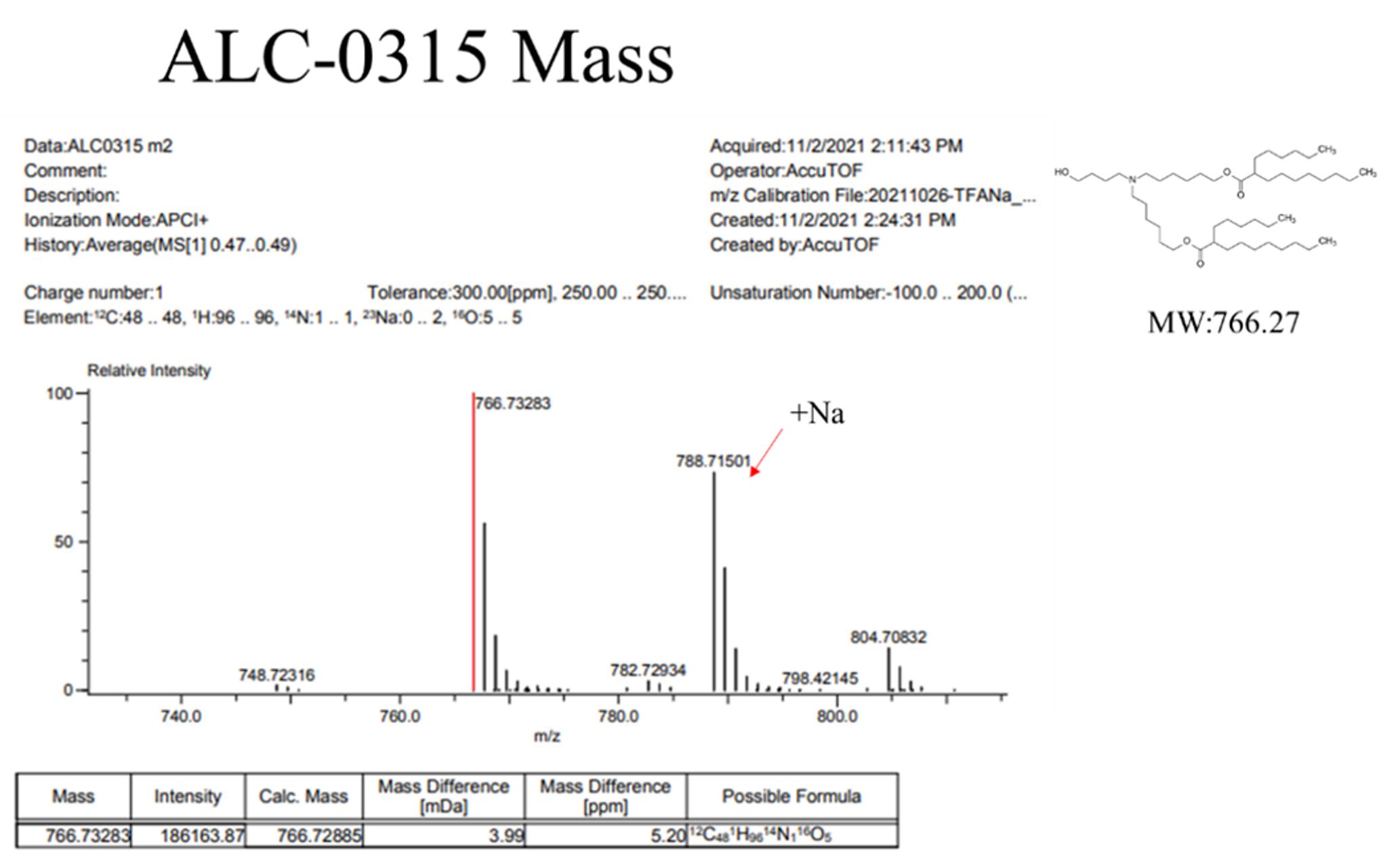
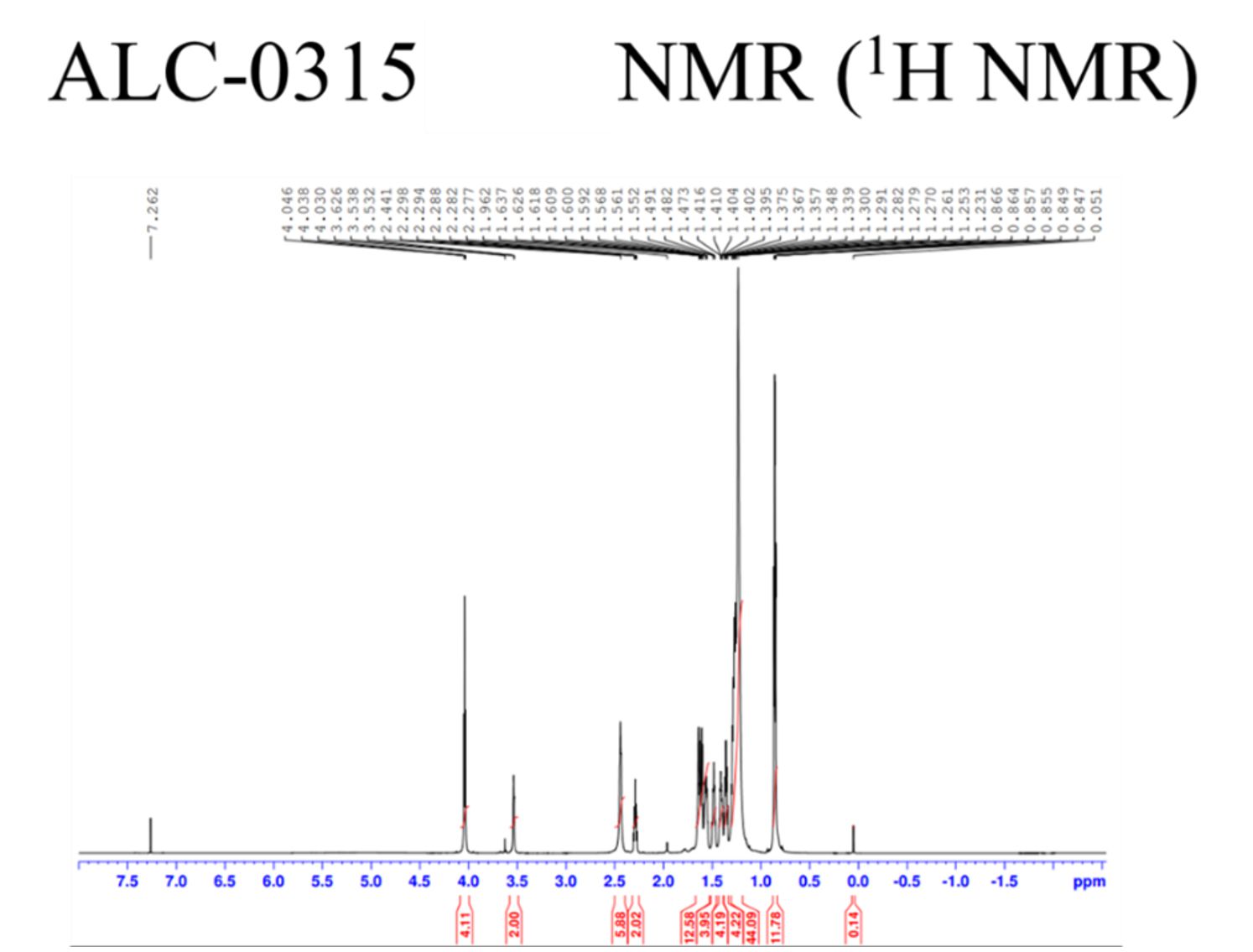
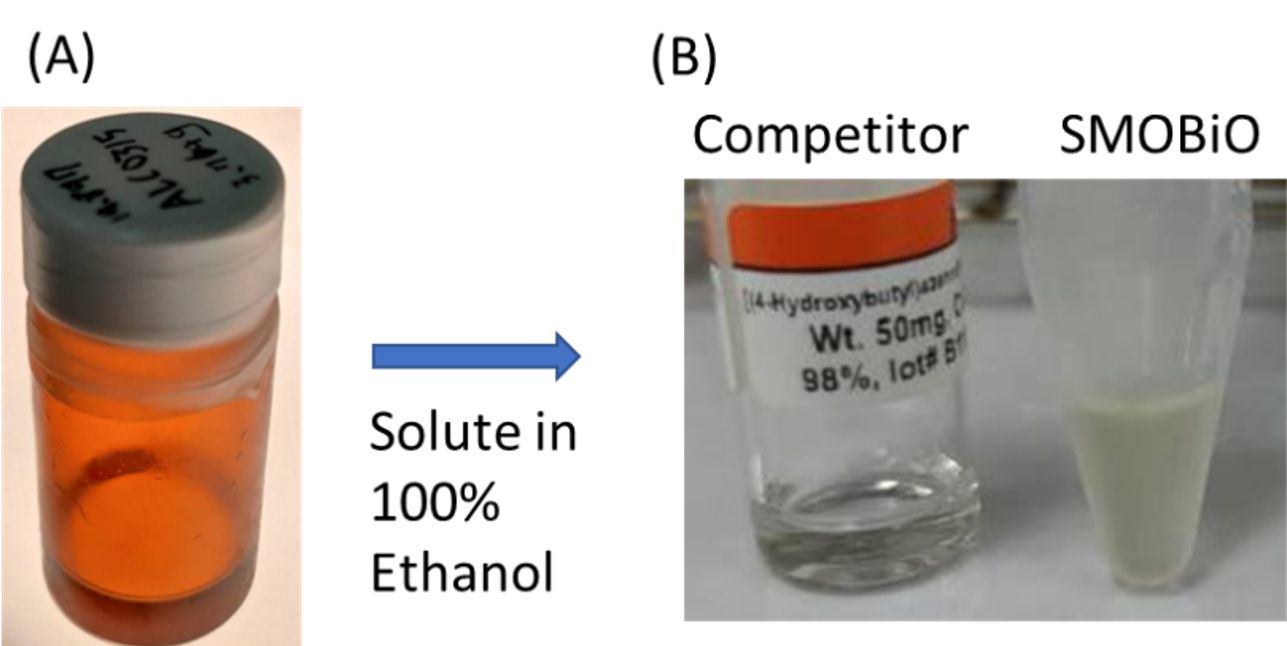
In-house synthesized ALC0315 lipid were presented as (A) original form and (B) dissolved in ethanol.
(2)mRNA-LNP preparation by using microfluidic system




