3. mRNA biologics and cold chain storage
1. mRNA lipid nanoparticle (LNP) biologics
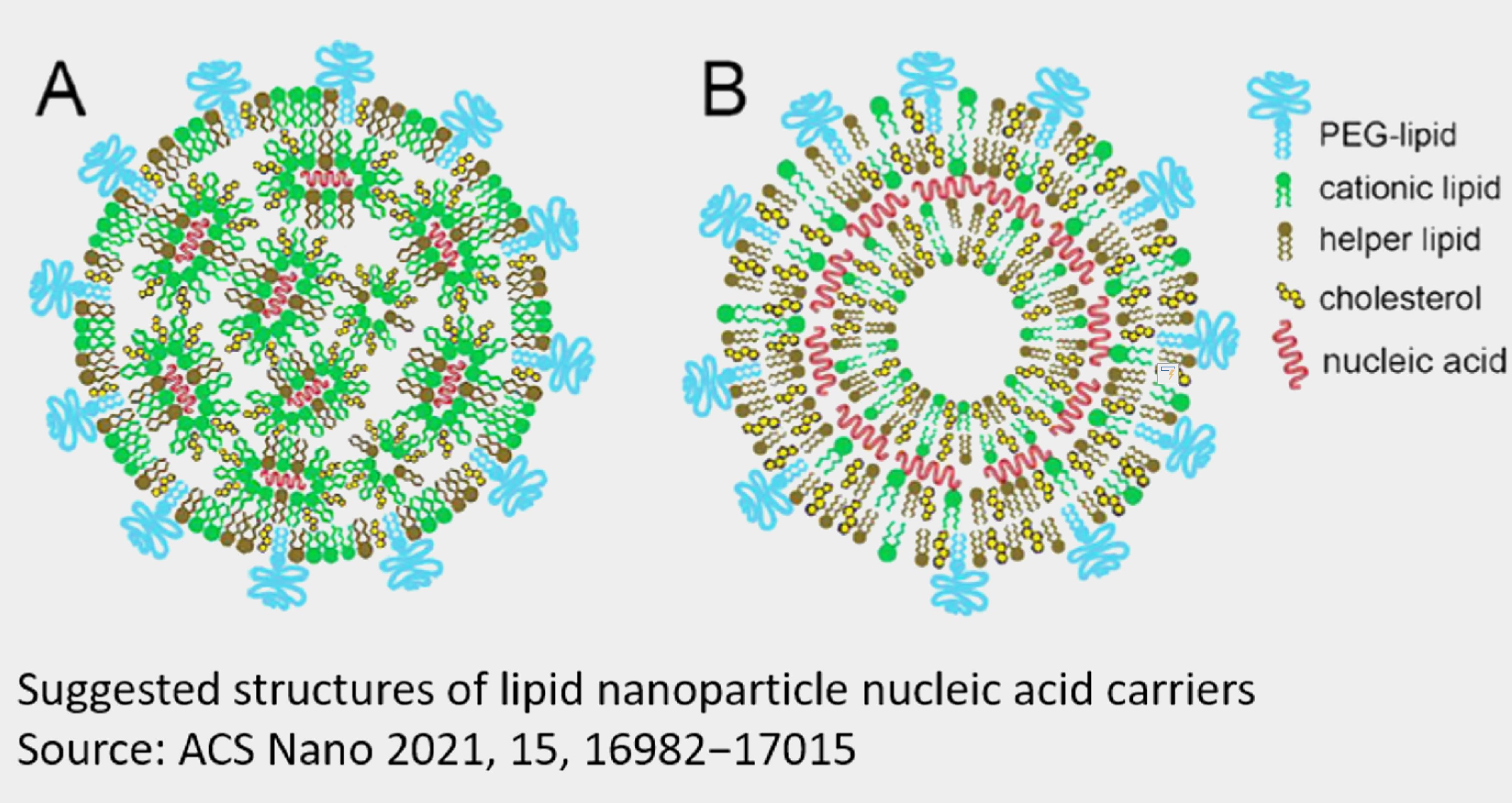
Another challenge coming after mRNA manufacturing is its delivery system. Compared to protein vaccine, RNA vaccine is relatively unstable and it shows function only after being securely delivered into human cells and therefore encoded proteins are generated inside, while protein vaccines are only to be injected into human body. Thus, the delivery system is one of key attribute for the success of mRNA vaccine. In other words, there wouldn’t be a successful mRNA vaccine if it was without LNP delivery system. Despite of the different compositions of LNP among biotech companies, the main components are introduced below:
Cationic lipid:
In order to form mRNA-LNP, negative charge of mRNA would be mixed with cationic lipid under certain pH environment, given the property of electrostatic interaction between two of them. After entering cell by endocytosis, LNP could subsequently be disrupted when cationic lipid binds to endosomal membrane, then mRNA would be released into cytoplasm. Therefore, cationic lipids are the most critical component in lipid micelle and have broad application prospects. However, some cationic lipids are cytotoxic because of cation in hydrophilic moiety. How to handle with human safety issue when using cation lipid is the core value for LNP delivery system, which also be the key claim of related patents.
PEG-lipid
:
PEG-labeling lipid could stabilize LNP structure by forming hydrophilic protection layer. PEG would mask LNP surface, which prevents from plasma protein binding and eradication thereby prolongs circulation time in human body
.
Cholesterol:
It provides membrane liquidity or rigidity, which increases the stability of LNP structure.
Helper lipid :
Distearoylphosphatidylcholine (DSPC), also named as helper lipid, which helps to release mRNA in endocytosis.

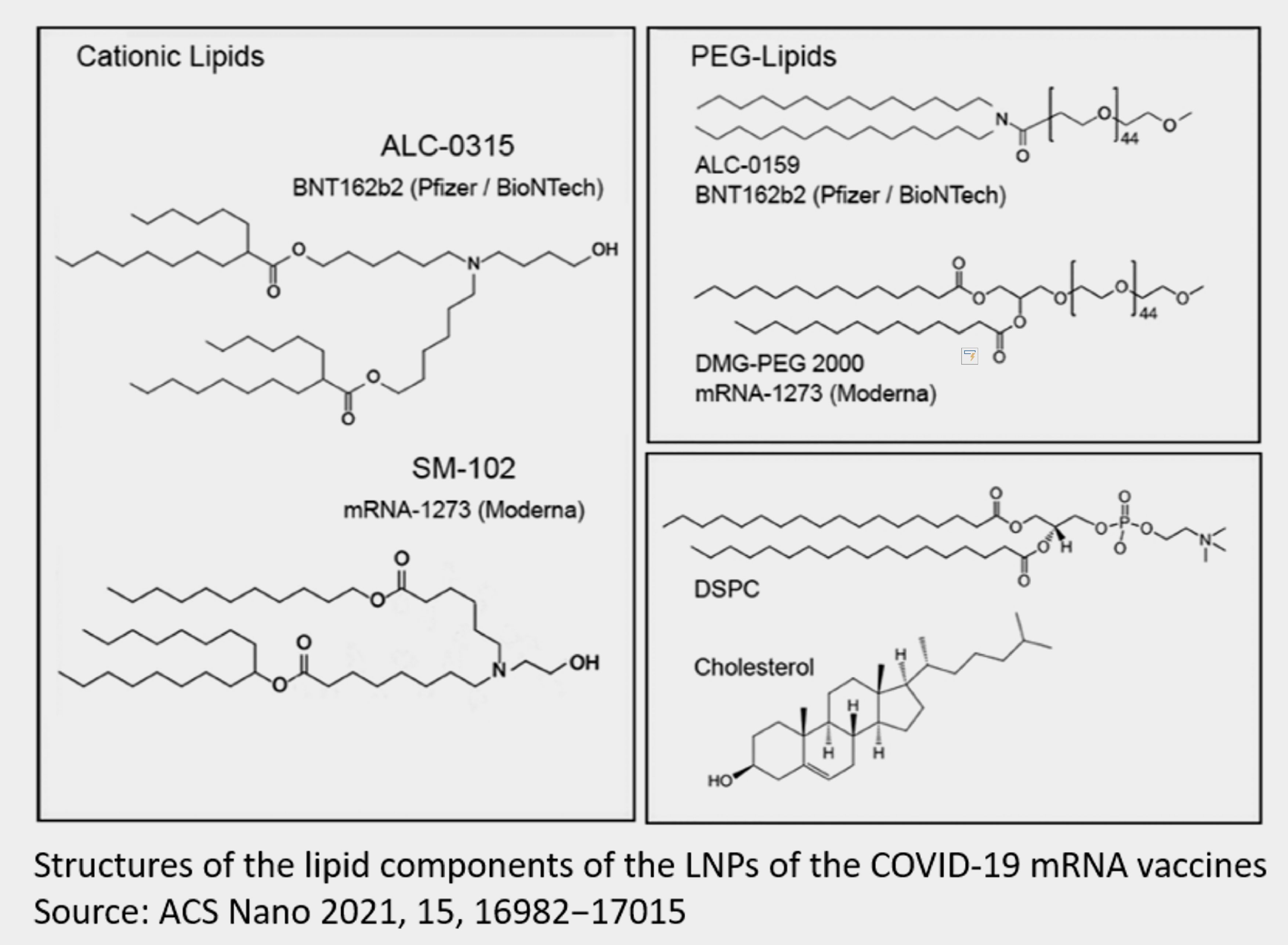
LC-0315 is the cationic lipid used for Pfizer/BioNTech mRNA vaccine. SMOBIO has successfully synthesized 7 grams of cationic lipid ALC-0315 for function validation use in October 2021 initially. The second test batch has enlarged 50-fold and achieved 350 grams of ALC-0315 in March 2022. We have planned to start kilograms batch production at Hsinchu scientific park. SMOBIO also has accomplished a test batch synthesis of SM-102 cationic lipid, which is used in Moderna mRNA vaccine. Lipid nanoparticle production is also a key part for making mRNA vaccine. Currently, the major industrial scale-up formulation is taken on microfluidic-based mixing. Based on this way, lipid dissolved in organic solvent will be injected from one channel and mix with nucleic acid in water that coming from the other channel, where are all controlled by microfluidic system. Parameters such as flow rate, ratio of injection volume, and cross-sectional area of channel are all critical determinants for final particle size and distribution of the LNP. It has been revealed that particles with diameter around 100 nm have better delivery efficiency. Quality control of mRNA-LNP includes encapsulation rate, pH of mRNA solution, pH of mRNA-LNP product, average particle diameter and distribution, particle micro-morphology, zeta potential, leakage and release profile analysis…etc. Encapsulation rate and mRNA quantity could be examined by 260 nm absorbance or fluorescent analysis detection. Cryo-EM can be applied for LNP morphology analysis and diameter measurement. Zeta potential and particle size measurement is achieved by DLS & zeta potential analyzer. High-performance liquid chromatography (HPLC) can be used for characterizing and quantifying LNP components.
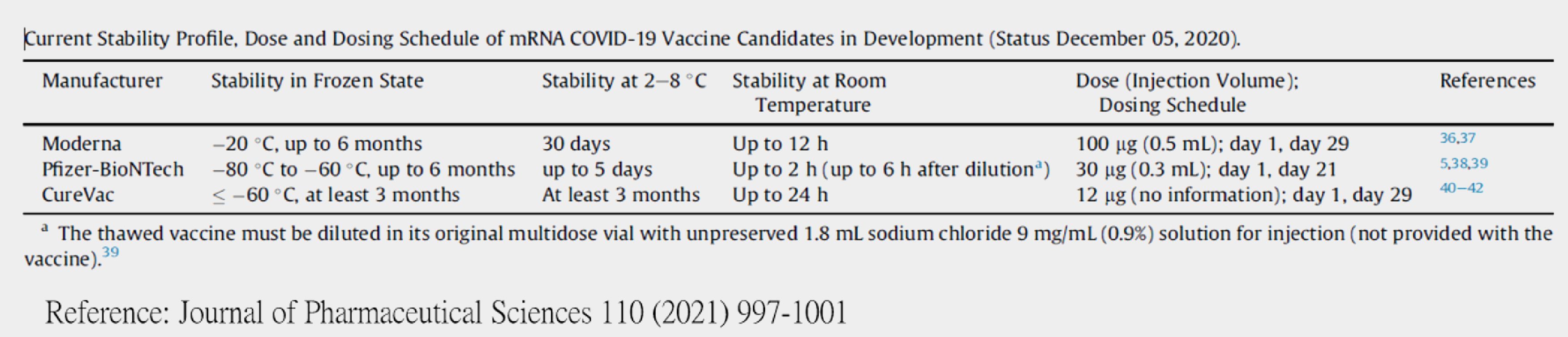
2. Cold chain storage
Since mRNA is temperature-sensitive, preservation time decreases with elevated temperature. It’s the pain point and requires ultra-low temperature during both shipping and storage by far. The following table summarizes the shipping and storage condition from all current mRNA vaccine companies.