Precast Gel Related Questions
How to choose appropriate Q-PAGE™ for my experiment?
What electrophoresis tanks are Q-PAGE™ Precast Gels compatible with?
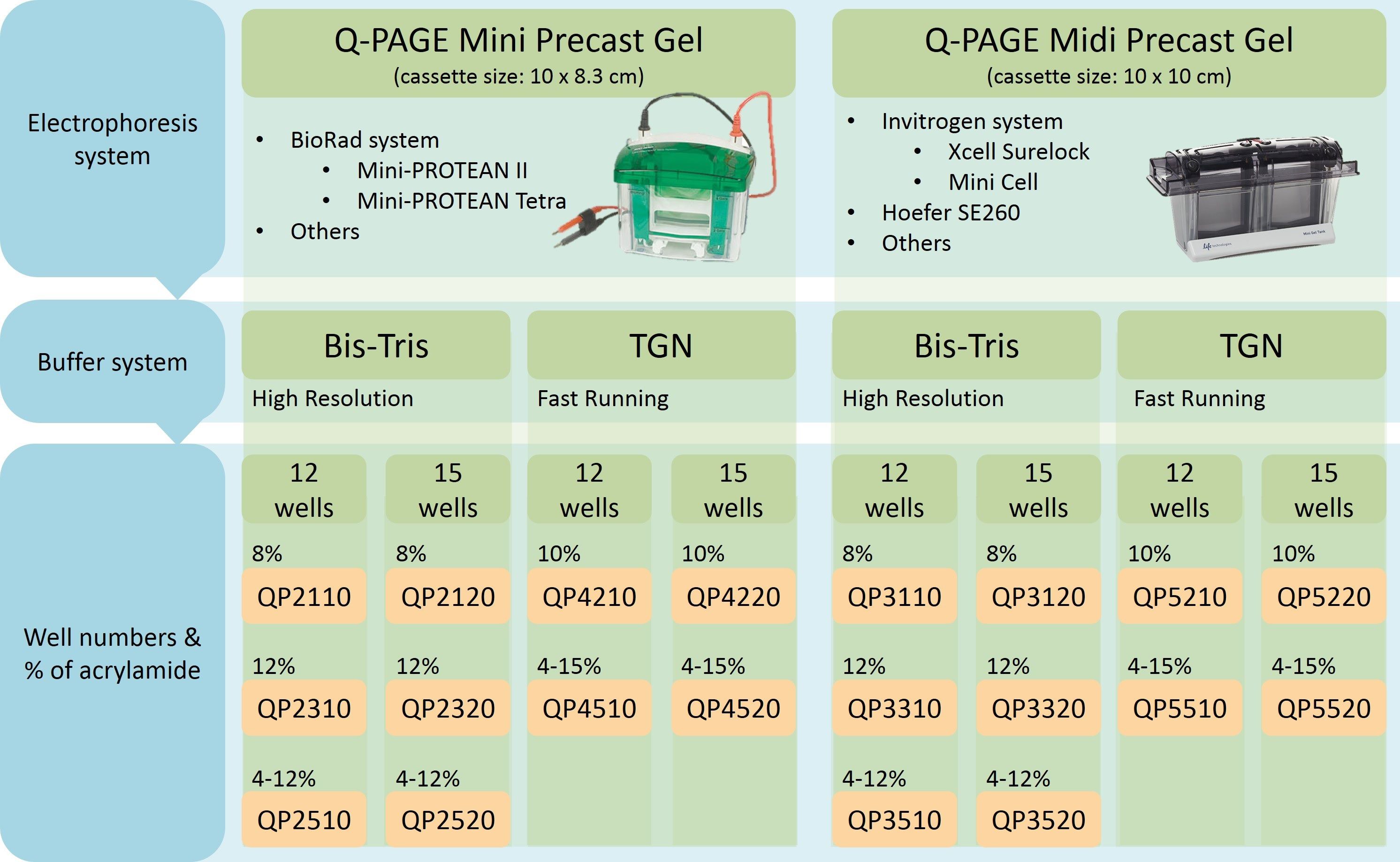
Q-PAGE™ Precast Gels are available in two cassette sizes, Mini (10 x 8.3 cm) and Midi (10 x 10 cm).
Q-PAGE™ Mini Gels (10 x 8.3 cm) are suitable for Bio-Rad® and other systems.
Q-PAGE™ Midi Gels (10 x 10 cm) are suitable for Invitrogen® XCell SureLock® Mini-Cell, Hoefer SE260, and other systems.
What percentage of acrylamide are available with Q-PAGE™ Precast Gels? How many sample wells?
Q-PAGE™ TGN Precast Gels are available in gradient (4 to 15%) and fixed (10%) concentrations of polyacrylamide in 12-well and 15-well formats.
Q-PAGE™ Bis-Tris Precast Gels are available in gradient (4 to 12%) and fixed (8% and 12%) concentrations of polyacrylamide in 12-well and 15-well formats.
What is the sample volume of the wells?
|
Gel Type |
Bis-Tris |
TGN (Tris-Glycine-Novel) |
||||||
|
Buffer systems |
MOPS and MES |
Tris-Glycine (Laemmli) |
||||||
|
Features |
Clear and sharp bands, high resolution |
Quick running, clear bands |
||||||
|
Cassette size |
Mini Gel |
Midi Gel |
Mini Gel |
Midi Gel |
||||
|
Electrophoresis system |
Bio-Rad systems |
Mini Gel Tank Xcell SureLock, Hoefer SE260 |
Bio-Rad systems |
Mini Gel Tank XCell SureLock, Hoefer SE260 |
||||
|
Well format & Capacity |
12 wells, 25 μ l/well |
15 wells, 22 μ l/well |
12 wells, 40 μ l/well |
15 wells, 28 μ l/well |
12 wells, 25 μ l/well |
15 wells, 22 μ l/well |
12 wells, 40 μ l/well |
15 wells, 28 μ l/well |
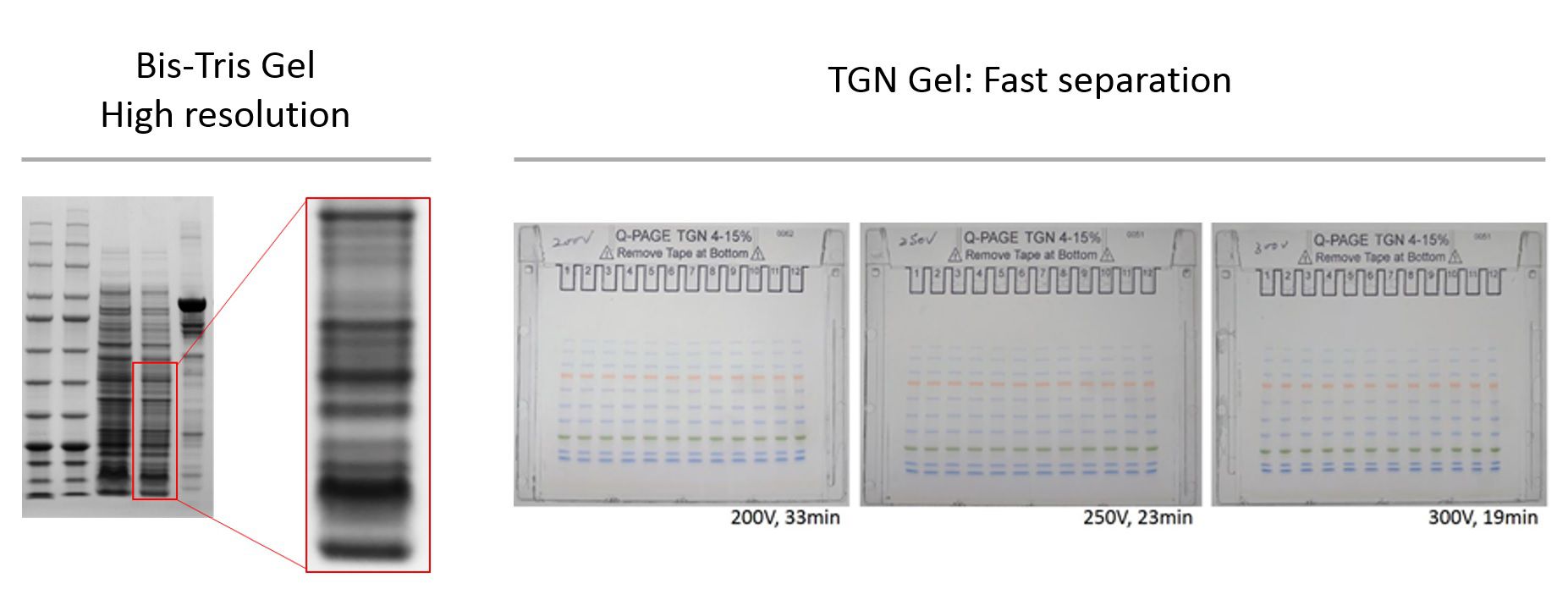
What is the difference between Bis-Tris Gels and TGN Gels?
Q-PAGE™ Bis-Tris Precast Gel features high resolution of protein separation and sharp bands.
Q-PAGE™ TGN Precast Gels features fast separation of protein samples.
What kind of running buffer can I use with Q-PAGE™ TGN Precast Gels? Can you provide recipe?
1. Conventional Tris-Glycine running buffer is suitable for Q-PAGE™ TGN Precast Gels.
2. 10X Tris-Glycine running buffer recipe:
Tris base 30.0 g, Glycine 144.0 g, SDS 10.0 g.
Bring up the volume to 1 L with ddH2O and dilute to 1X for use.
What kind of running buffer can I use with Q-PAGE™ Bis-Tris Precast Gels? Can you provide recipe?
MOPS or MES running buffer is suitable for Q-PAGE™ Bis-Tris Precast Gels.
10X MOPS running buffer recipe:
Tris base 60.6 g, MOPS 104.6 g, SDS 10.0 g, EDTA 3.0 g.
Bring up the volume to 1 L with ddH2O and dilute to 1X for use.
10X MES running buffer recipe:
Tris base 60.6 g, MES 97.6 g, SDS 10.0 g, EDTA 3.0 g.
Bring up the volume to 1 L with ddH2O and dilute to 1X for use.
Do not use Tris-Glycine running buffer for Q-PAGE™ Bis-Tris Precast Gels.
What buffer can I use to transfer Q-PAGE™ Precast Gels? Can you provide recipe?
Tris-Glycine based transfer buffer is suitable for both Q-PAGE™ TGN and Bis-Tris Precast Gels.
Towbin Buffer (for wet transfer)
10X buffer recipe:
Tris base 30.0 g, Glycine 144.0 g. Bring up the volume to 1 L with ddH2O.
Dilute 10X transfer buffer to 1X for use:
100 ml 10X transfer buffer, 700 ml ddH2O, and 200 ml methanol
*Add SDS to 0.01 ~ 0.1% to promote transfer of high molecular weight proteins.
**Cool 1X transfer buffer to 4°C before using.
Bjerrum Schafer-Nielsen Buffers (for semi-dry transfer)
10X buffer recipe:
Tris base 58.15 g, Glycine 29.3 g. Bring up the volume to 1 L with ddH2O.
Dilute 10X transfer buffer to 1X for use:
100 ml 10X transfer buffer, 700 ml ddH2O, and 200 ml methanol
*Add SDS to 0.01 ~ 0.1% to promote transfer of high molecular weight proteins.
There is a white mark on the left side of the cassette, will it affect the precast gel quality?
The white mark is naturally generated when two plastic plates are pressed together during production, and will not affect the gel performance or subsequent analyses.
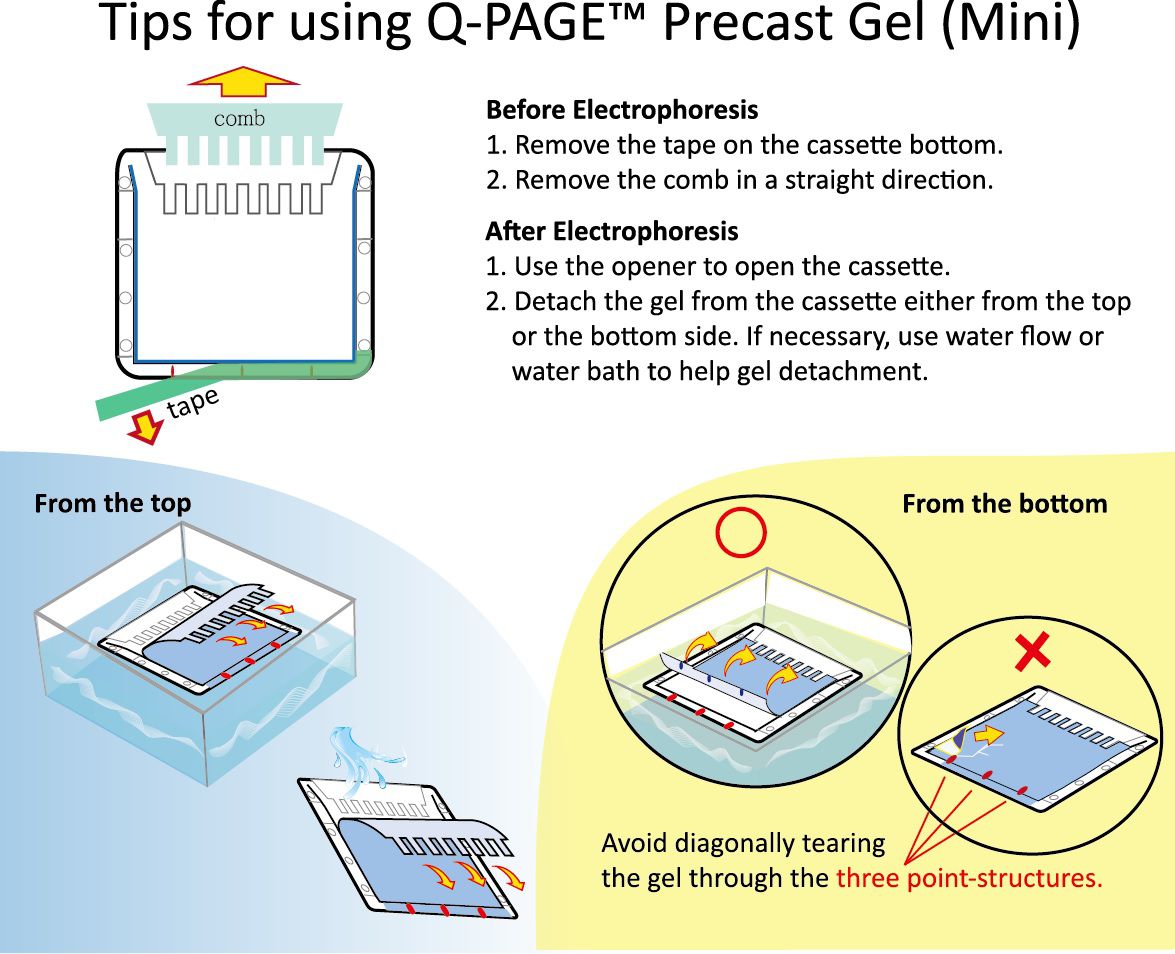
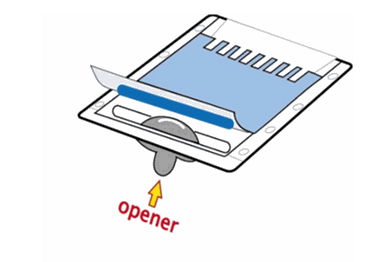
How can I open the cassette (Mini) and remove Q-PAGE™ Precast Gel from cassette? Do you provide helpful tools?
Open cassette immediately after electrophoresis. Avoid gel drying.
1. Insert the cassette opener into corners of cassette.
2. Sequentially pry the opener to separate the two plates.
3. Gently pull two plates apart from the top of cassette.
4. Carefully detach the gel either from the bottom or the top side of the cassette.
‒ Avoid diagonally peeling the gel from the corner.
‒ Use water and remover to help gel detachment if needed.
5 . Gently remove the gel for further staining or Western blotting.
How can I open the cassette (Midi) and remove Q-PAGE™ Precast Gel from cassette? Do you provide helpful tools?
Open Gel Cassette and remove Q-PAGE™ Midi Gel
Open cassette immediately after electrophoresis. Avoid gel drying.
1. Insert the cassette opener into corners of cassette.
2. Sequentially pry the opener to separate the two plates.
3. Gently pull two plates apart from the top of cassette.
4. Use cassette opener to push through the slot in the cassette.
5. Carefully detach the gel from the bottom side of gel.
‒ Avoid diagonally peeling the gel from the corner.
‒ Use water and remover to help gel detachment if needed.
6. Gently remove the gel for further staining or Western blotting.
Tips for using Q-PAGETM Precast Gel (Midi)
Before Electrophoresis:
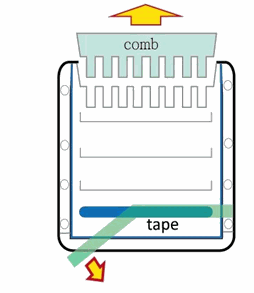
1. Remove the green tap on the cassette.
2. Remove the comb in a straight direction.
After Electrophoresis:
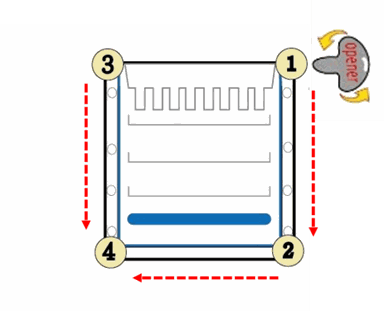
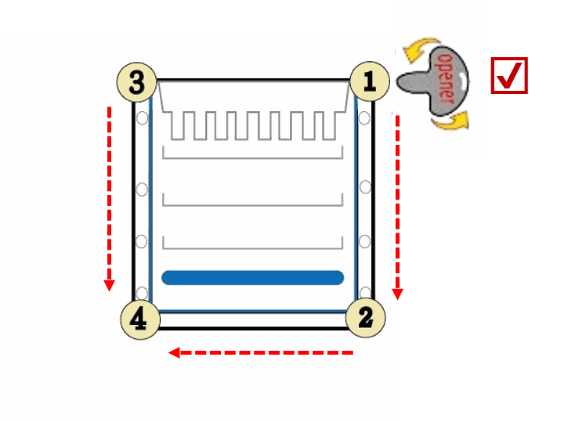
1. Separate each of the three bonded sides of the cassette by inserting the cassette opener into the gap between the two plastic plates and twisting carefully. Start from the corner at the top, and move along each side of the cassette. For example: Start moving from corner ① to ②, moving from corner ③ to ④, and then moving from corner ② to ④.
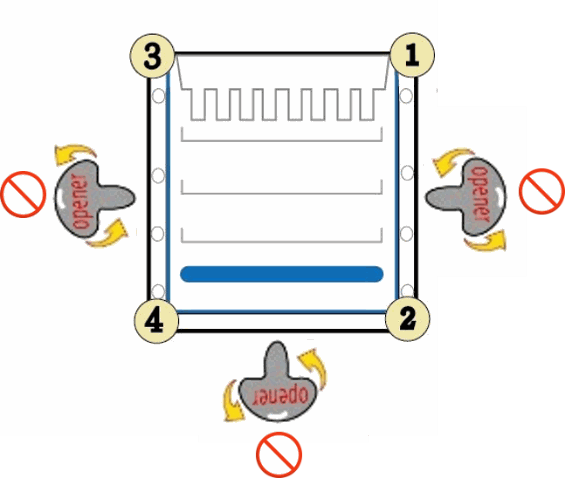
Caution: Do not directly open the cassette from the middle of bonded sides of cassette.
2. Gently pull two plates apart from the top of cassette until the plates are completely separated.
How to avoid plate breaking when opening the cassette of Q-PAGE™ Gel (Midi)?
1. Do not directly open the cassette from middle of bonded sides of cassette. Doing so has risk to cause plates to break.
2. To correctly open the cassette, separate each of the three bonded sides of the cassette by inserting the cassette opener into the gap between the two plastic plates from the corner and twisting carefully. Start opening from the corner at the top, and move along each side of the cassette. For example: Start moving from corner ① to ②, moving from corner ③ to ④, and then moving from ② to ④.
Why do I occasionally see bubbles between the gel and the plate before or after running the gel? Will this impact the results?
The air bubble may occasionally be seen in the precast gel before use, particularly at the lateral edges between the gel and the plate, but not within the gel itself. These bubbles typically do not interfere with the SDS-PAGE process, because the whole gel matrix remains intact and therefore your results will remain unaffected.
Similarly, during electrophoresis, some small bubbles might appear along with the migration front, which also are normal phenomena and do not interfere the results.
Will the updated bottom sealing tape of the Q-PAGE™ precast gel affect usage? Is there anything I should be aware of?
We’ve upgraded the bottom sealing tape of the Q-PAGE™ precast gel to enhance user experience. The new version offers:
-
Improved visibility: The lime green tape now has reduced translucency, providing better contrast for easier identification and handling.
-
Stronger adhesion: Better adhesion ensures a more secure seal.
Due to the increased adhesive strength, a small amount of residue may occasionally remain after tape removal. This does not affect gel performance or experimental results, and no additional cleaning is necessary.
Troubleshooting Guidelines
Well deformation, bubbles between gel and cassette, or gel leaked out of cassette
Possible Cause:
Gel has been frozen or stored at wrong temperature.
Suggested Solution:
Store Q-PAGE™ Precast Gels at 4°C.
Well separators are twisted or crooked after removing comb.
Possible Cause:
Pull the comb out too slowly and not in a straight direction.
Suggested Solution:
Pull the comb straightly and quickly out of the cassette to prevent well distortion. Use long loading tip or syringe needle to correct the position of well separators.
Buffer leaking from the inner chamber
Possible Cause:
Untight assembly of gels to the electrode modules
Suggested Solution:
Reassemble Q-PAGE™ gels into the electrode modules.
Fill outer chamber with 1X running buffer to the highest level.
Samples do not sink into the wells.
Possible Cause:
1. Residual gel storage buffer in the wells
2. Insufficient sample buffer
Suggested Solution:
1. Rinse the gel wells with ddH
2
O or 1X running buffer before sample loading.
2. Use more sample buffer to prepare samples.
Current is zero or less than expected and sample do not migrate into gel.
Possible Cause:
1. Tape at bottom of cassette not removed
2.
Cassette is put in an opposite position in the Invitrogen Mini Gel Tank
Suggested Solution:
1. Remove tape
2.
The shorter side of the cassette must face outward in Invitrogen Mini Gel Tank as figure shown below
Gels run faster or more slowly than expected.
Possible Cause:
Incorrect running buffer
Suggested Solution:
Check buffer composition.
Use fresh 1X running buffer for inner chamber.
Crooked bands at middle or bottom of gel
Possible Cause:
1. Gel has been frozen or stored at wrong temperature.
2. Incorrect running buffer
Suggested Solution:
1. Store Q-PAGE Precast Gels at 4°C.
2. Check buffer composition. Use fresh 1X running buffer for inner chamber.
Band pattern curves toward one side of gel
Possible Cause:
1. Buffer leaking from the inner chamber
2. Excessive heating of gel
3. Insufficient buffer in inner or outer buffer chamber
Suggested Solution:
1. Check assembly of gels into the electrode modules.
2. Check buffer composition. Or dilute running buffer to 0.5-0.75X. Do not exceed recommended running conditions.
3. Fill inner and outer chambers to completely cover gel wells.
Band pattern curves toward one or both sides of gel
Possible Cause:
1. Buffer leaking from the inner chamber
2. Excessive heating of gel
3. Insufficient buffer in inner or outer buffer chamber
Suggested Solution:
1. Check assembly of gels into the electrode modules.
2. Check buffer composition. Or dilute running buffer to 0.5-0.75X. Do not exceed recommended running conditions.
3. Fill inner and outer chambers to completely cover gel wells.
Poor resolution or fuzzy bands
Possible Cause:
1. Excessive heating of gel
2. Incorrect running buffer
Suggested Solution:
1. Check buffer composition. Use fresh 1X running buffer for inner chamber.
2. Check buffer composition.
Bands are missing on the membrane after Western transferring.
Possible Cause:
Proteins move in the wrong direction
Suggested Solution:
Check the order of gel/membrane sandwich assembly, the direction of transfer cassette in transfer modules, and the polarity of connections to power supply.
Swirls or missing bands; bands trail off in multiple directions on the membrane after Western transferring.
Possible Cause:
Contact between the membrane and the gel was poor; Air bubbles or excess buffer remains between the blotting membrane and the gel.
Suggested Solution:
Use thicker/more filter paper in the gel/membrane sandwich. Remove air bubbles and excess buffer between gel and membrane by carefully moving the roller over the membrane.
Apparent molecular sizes of prestained protein markers are different as indicated.
Possible Cause:
Prestained protein markers used have not been calibrated
for use with Q-PAGE gels. Dyes for staining protein markers affect the migration patterns of prestained proteins in different buffer systems.
Suggested Solution:
Calibrate prestained protein markers against unstained proteins of known size or use SMOBIO’s ExcelBand™ Protein Markers.
Buffer temperature hotter than expected when running gel
Possible Cause:
Incorrect running buffer
Suggested Solution:
A. Check buffer composition.
B. Use fresh 1X running buffer for inner and outer chamber.
The buffer has leaked when put Q-PAGE™ TGN Midi Precast Gel in the device Hoefer SE260.
Possible Cause:
1. Dislocation of the gel cassette in the electrophoresis tank.
2. Looseness of the spring in spring clamps.
3. Dirt, nicks or abrasion on the gray silicone rubber gasket.
Suggested Solution:
1. Place the gel cassette directly down to the bottom of the electrophoresis tank.
The bottom edge of the gel cassette should be completely against the bottom of the electrophoresis tank.
2. Replace it with a new spring clamp.
3. Clean away any gel remains adhering to the foam gasket. Remove the gray silicone rubber gasket from the core. If there is nicks or abrasion, replace it with a new one.