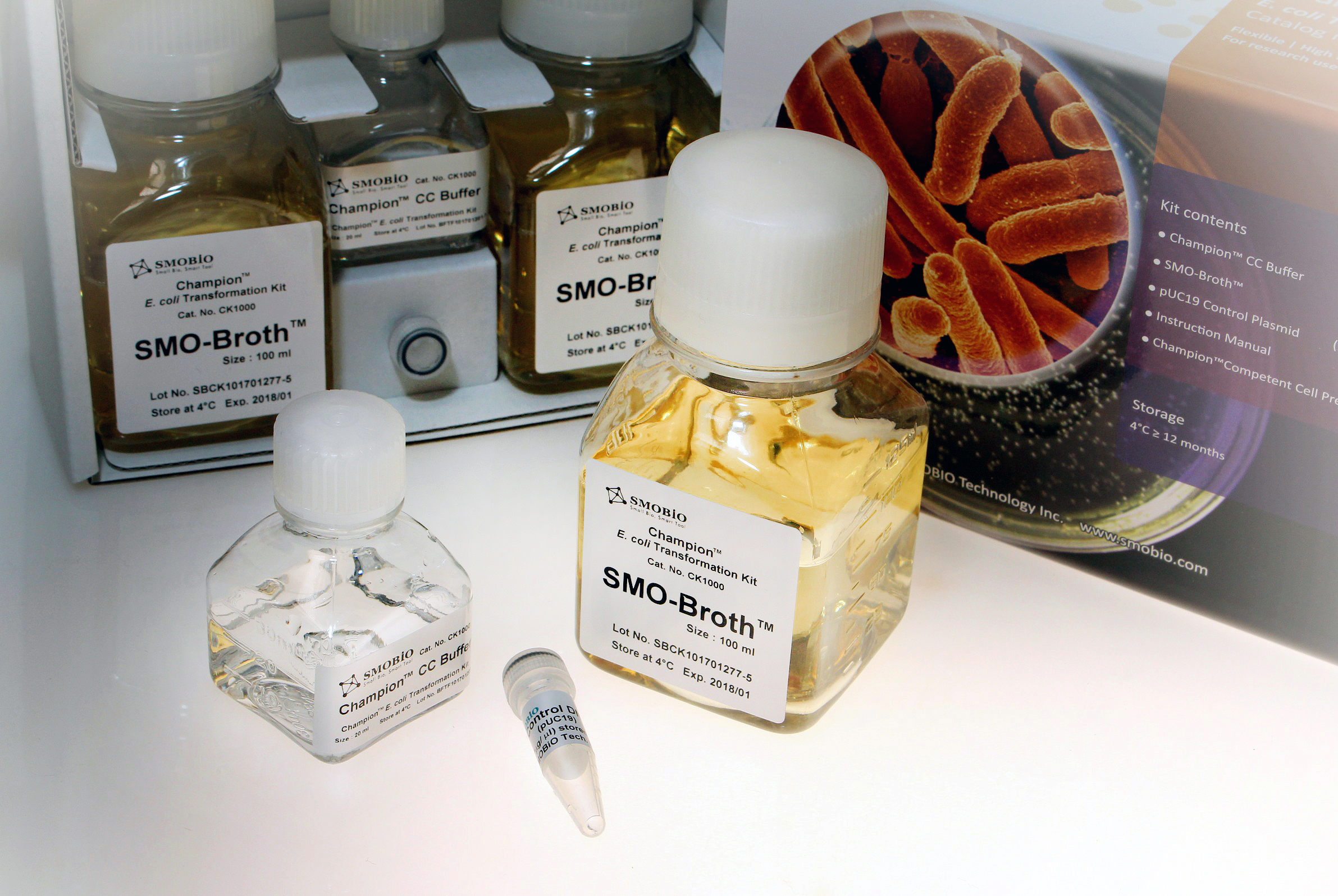
Description
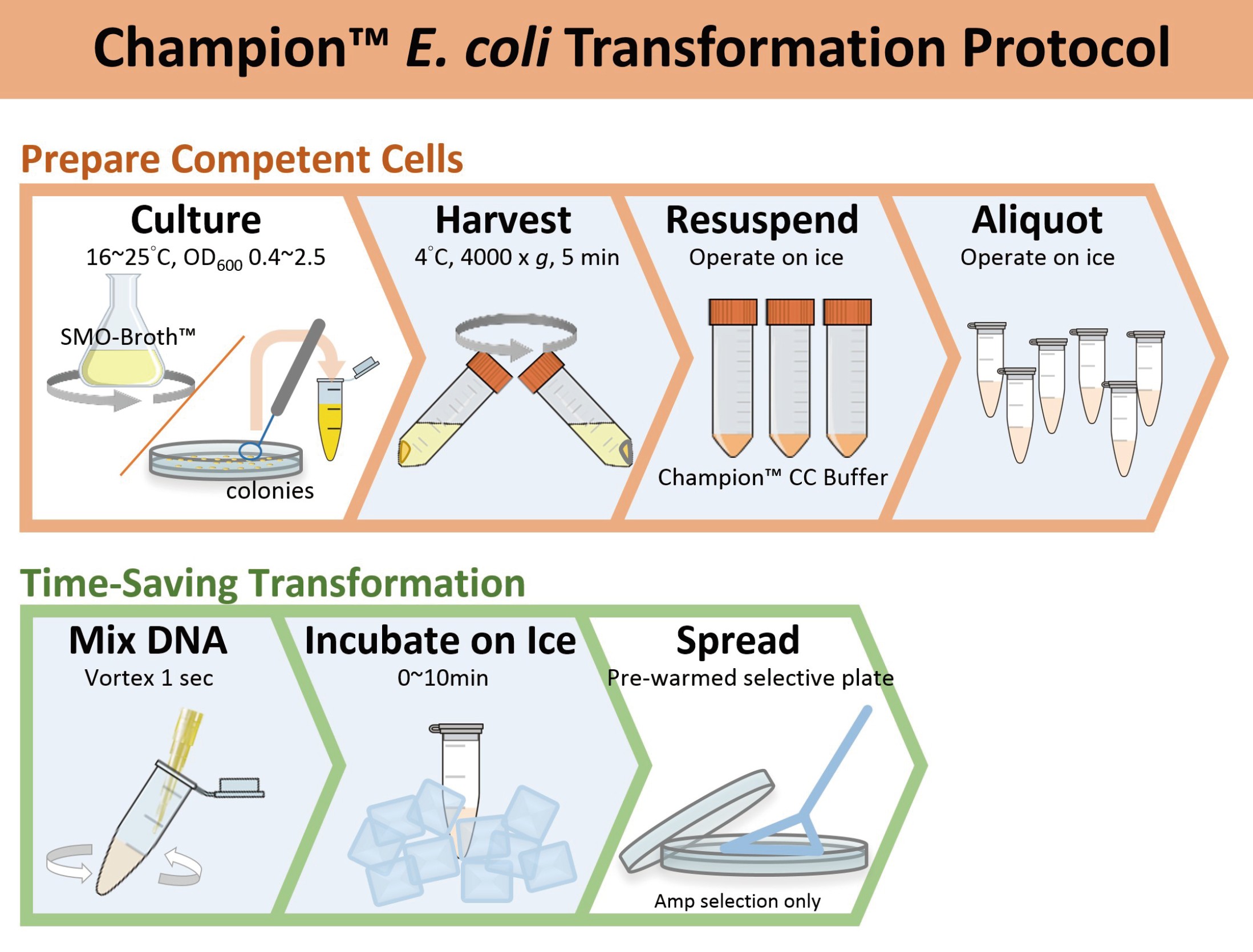
Champion™ E. coli Transformation Kit provides an easy method for rapid preparation of chemically competent cells with high transformation efficiency from fresh culture, overnight culture, or even directly from bacterial colonies on the plate. The competent cell preparation method eliminates the requirement of time-wasting wash step. In addition, preparation of competent cells from overnight culture or directly from bacterial colonies provides flexibility to cloning experiment. The resultant competent cells can be immediately used or stored at -70°C for one year. This kit includes a specialized SMO-Broth™ medium and a unique Champion™ CC Buffer for culturing and preparing competent cells efficiently. Following the simple and quick competent cell preparation protocol from fresh culture, the transformation efficiency is typically ranged from 108–109 cfu/μg transformants/μg of pUC19 plasmid DNA, but varies depending on the E. coli strains. The resultant competent cells can be further transformed using time-saving transformation protocol, eliminating the requirement of heat-shock and recovery steps.
Features
Flexible– fresh culture, overnight culture, 4°C stored liquid culture or even colonies on agar plate can be used for transformation.
Fast and Easy– only few steps for preparation; suitable for time-saving transformation
High efficiency– up to 109 cfu/μg
Personalization– suitable for most E. coli strains
Kit Contents
|
Component
|
Volume
| |||||
|
Champion™ CC Buffer
|
20 ml
| |||||
|
SMO-Broth™
|
100 ml x 2
| |||||
|
pUC19 Control Plasmid (10-4 μg/μl)
|
5 µl
| |||||
|
Instruction Manual
|
1
| |||||
|
Champion™ Competent Cell Preparation Card
|
1
|
Storage
4°C for 12 months
Prepare Competent Cells with High Transformation Efficiency Using Champion™ E.coli Transformation Kit
Prepare Competent Cells Directly from E.coli Colonies Using Champion™ E.coli Transformation Kit
Time-saving Transformation Protocol
Kit Contents
|
Component |
Volume |
|
Champion™ CC Buffer |
20 ml |
|
SMO-Broth™ |
100 ml x 2 |
|
pUC19 Control Plasmid (10-4 μg/μl) |
5 µl |
|
Instruction Manual |
1 |
|
Champion™ Competent Cell Preparation Card |
1 |
Storage 4°C for 12 months |
Can Champion™ E. coli Transformation Kit be used to make competent cell of other bacteria?
Champion™ E. coli Transformation Kit are specially designed for making E.coli competent cells. Therefore, Champion™ E. coli Transformation Kit is not recommend to used for make other bacterial competent cells.
What E.coli. strain is compatible to Champion™ E. coli Transformation Kit?
Most E.coli strains are compatible to Champion™ E. coli Transformation Kit. However, different E. coli strains vary in their ability to be transformed with DNA.
Efficiency of strains and their derivatives is listed below, when prepared with the Champion™ E. coli Transformation Kit.
E. coli strains | Efficiency (cfu/μg) |
JM109 | ~5x108 |
XL-1 blue | ~3x108 |
DH5α | ~5x108 |
stbl 3 | ~2x108 |
BL21 | ~1x107 |
Rosetta 2 | ~1x107 |
*E. coli strains are liquid cultured following standard protocol for preparation of competent cells (at 25°C until OD600 reached ~0.5).
Will transformation efficiency be reduced when Champion™ competent cells are thawed, dispensed and refrozen repeatedly?
When Champion™ competent cells are thawed dispensed in aliquots and refrozen within 1 min, the transformation efficiency will be 10~20% reduced compared to the first time use.
What is the advantage of thawing competent cells with circulating water instead of still water?
Using circulating water can reduce the thawing time. Less thawing time shows higher efficiency than long thawing time.
Is there any difference of transformation efficiency between using plating beads, bending glass rod and plating loop?
The bending glass rod shows best efficiency and the plating loop show less efficiency than other method. For handle a lot samples at the same time the plating beads are recommended.
Is 1 second vortex critical for mixture the DNA?
One second vortex provides more reliable transformation efficiency (1.1 times compare with mixed by finger tapping)
Will heat shock affect the transformation efficiency?
Heat shock treatment will enhance the efficiency about 1~2X versus non-heat shock method.
Is it necessary to change the transformation procedure for transforming a large plasmid?
For large plasmid (> 6 kb), the heat shock method will significantly improves the efficiency and the recovery procedure also significantly improves the efficiency.
How to reduce the interference of the satellite colonies?
Using proper antibiotics of suitable concentration or using fresh antibiotics.
Jin-Xuan Tian, Wen-Shi Tsai, I-Hsin Sung. Insects. 2023 Jan 18;14(2):103. doi: 10.3390/insects14020103.
PMCID: PMC9958760
Tips for Making High Competence of E. coli
Culture Condition
E. coli cells present high competence when freshly cultured from 1/100~1/20 dilution of overnight culture and incubated at 16~25°C prior to preparation. Higher temperature (37°C) leads to competence decrease.
|
Temperature |
OD600 |
Culture time |
Efficiency (cfu/μg) |
|
16°C |
0.4~0.8 |
12~20 hr |
~1x109 |
|
25°C |
0.4~0.8 |
3~8 hr |
~5x108 |
|
37°C |
0.4~0.8 |
1~1.5 hr |
~1x108 |
|
25°C |
1~2.5 |
4~10 hr |
~3x108 |
E. coli cells in liquid culture are higher competent than colonies on plate.
|
Culture Condition |
Efficiency (cfu/μg) |
|
Liquid culture (fresh or overnight culture) |
108~109 |
|
Fresh colonies (grown overnight at 37°C) |
~2x106 |
|
Old colonies (4°C stored for 7 days) |
~5x105 |
E. coli Strains
Different E. coli strains vary in their ability to be transformed with DNA. Efficiency of strains and their derivatives is listed below, when prepared with the Champion™ E. coli Transformation Kit.
|
E. coli strains |
Efficiency (cfu/μg) |
|
JM109 |
~5x108 |
|
XL-1 blue |
~3x108 |
|
DH5α |
~5x108 |
|
stbl 3 |
~2x108 |
|
BL21 |
~1x107 |
|
Rosetta 2 |
~1x107 |
*E. coli strains are liquid cultured following standard protocol for preparation of competent cells (at 25°C until OD600 reached ~0.5).
Storage Condition
To maintain high competence, E. coli should be stored at -70°C. Higher temperature (above -50°C) leads to competence decrease.
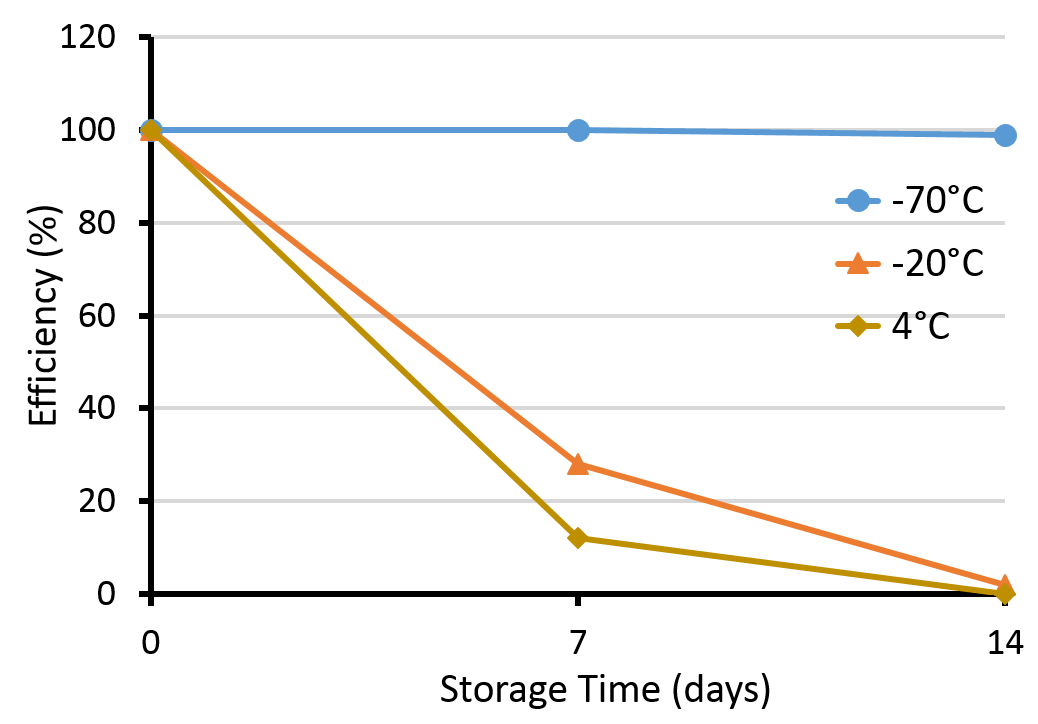
Factors Affecting Transformation Efficiency
Thawing methods
Shorter thawing time is more efficient than a longer thawing time. Slow thawing caused by power shortages and unstable freezers will result in decreased efficiency.
Size of plasmid
Plasmid size affect the efficiency greatly. The efficiency of transforming a supercoiled 2.7 kb is approximately 100 times higher than that of a 10 Kb plasmid (using time-saving transformation protocol). For large plasmids (> 6 kb), standard transformation protocol is recommended.
Heat shock treatment
Heat shock treatment will enhance the efficiency about 1~2 folds versus non-heat shock method.
Plating methods
Bent glass rods show the greatest efficiency, while plating loop shows less efficiency than plating beads. When handling a large quantity of samples at the same time, plating beads are recommended.
Concentration of antibiotic
Antibiotic concentration is critical to use of the time-saving transformation protocol.
|
Antibiotic |
Concentration |
|
Ampicillin (Ap) |
20 μg/ml |
|
Kanamycin (Km) |
25 μg/ml |
|
Tetracycline (Tc) |
7.5 μg/ml |
|
Chloramphenicol (Cm) |
20 μg/ml |
For plasmid size <6 Kb, the efficiency of kanamycin selection is usually 3~10 times less than the ampicillin selection. For plasmid size > 6 Kb, the efficiency of kanamycin selection is much lower than ampicillin. We suggest using the standard transformation protocol (with heat shock and recovery steps) to enhance the efficiency.
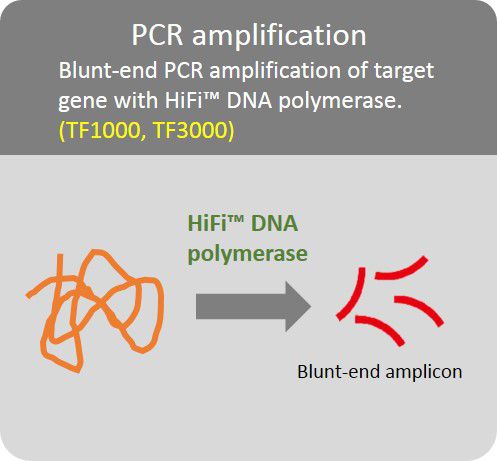
High Fidelity PCR amplification
Amplification of target gene with HiFi™ DNA polymerase to minimize error rate.

Gel electrophoresis
Staining amplicons with safe fluorescent dyes, following by observation under blue-light illuminator to minimize damage of DNA amplicons and maximize successful cloning efficiency.
Safe fluorescent dyes
[NS1000] FluoroVue™ Nucleic Acid Gel Stain (10,000X), 500 μl
[DS1000] FluoroStain™ DNA Fluorescent Staining Dye (Green, 10,000X), 500 μl
[DL5000] FluoroDye™ DNA Fluorescent Loading Dye (Green, 6X), 1 ml
Blue-light illuminator
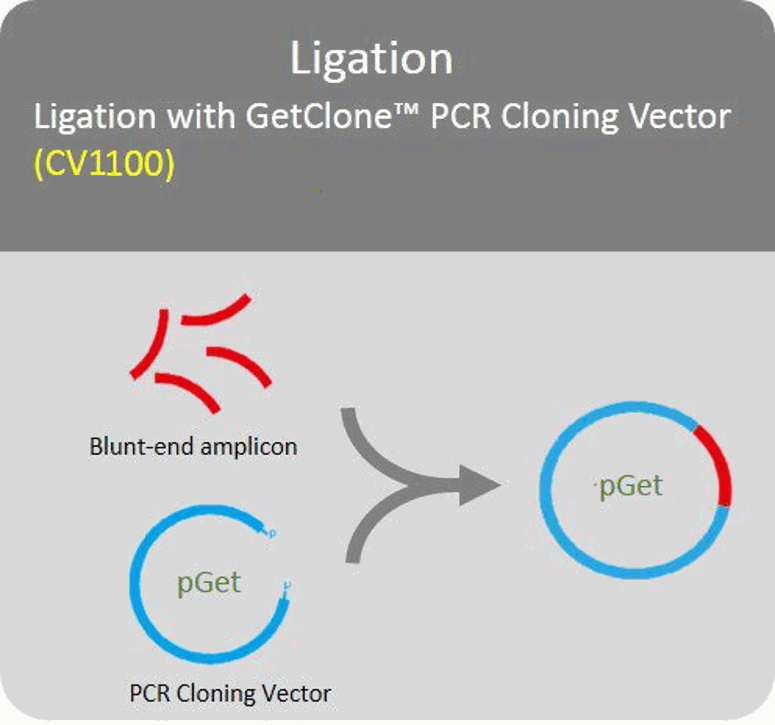
Ligation
Blund-end PCR amplicons can directly ligate with PCR cloning vector.
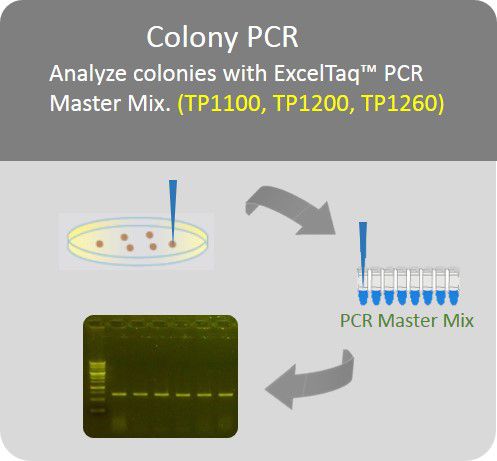
Colony PCR
Analyze colonies with PCR master mix to save preparation time.

![[CK1000] Champion™ E. coli Transformation Kit, 200 Rxn](/web/image/product.template/217/image?unique=a0e4253)
![[CK1000] Champion™ E. coli Transformation Kit, 200 Rxn](/website/image/product.template/217/image/90x90)


