Description
The FluoroStain™ DNA Fluorescent Staining Dye is designed to be a safer replacement for conventional Ethidium bromide (EtBr) which poses a significant health and safety hazard for its users. The FluoroStain™ DNA Fluorescent Staining Dye offers at least 10 times sensitivity in DNA detection levels, and is capable of detecting double stranded DNA (dsDNA) fragments up to 0.04 ng in electrophoresis analysis. The FluoroStain™ DNA Fluorescent Staining Dye shows a high specificity to the dsDNA, with negligible background signal, making the destaining process entirely optional. FluoroStain™ DNA Fluorescent Staining Dye is compatible with both the conventional ultra violet gel-illuminating systems as well as the less harmful long wave length blue light illumination systems. The emission when bound to dsDNA is 522 nm, while its excitation peaks are at 270, 370 and 497 nm.
Features:
Excellent for post staining
Sensitivity: 0.04 ng DNA
A safer alternative to EtBr
Compatibility: suitable to blue or UV light
Increased cloning efficiency (blue light)
Storage
Protected from light
4°C for 12 months
-20°C for 24 months
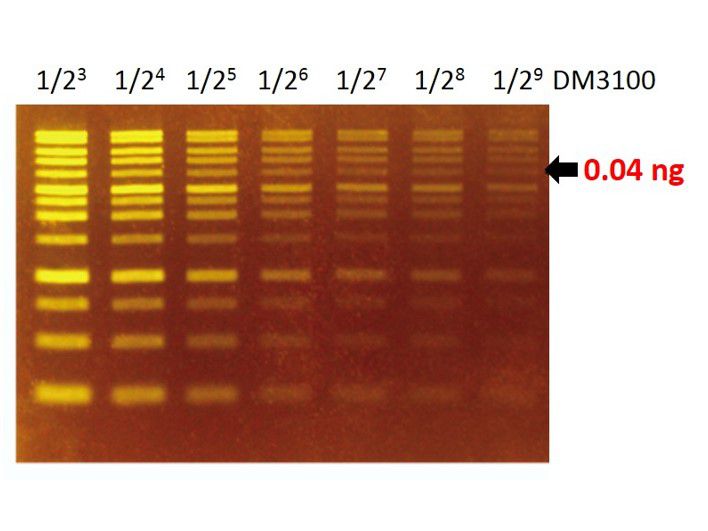
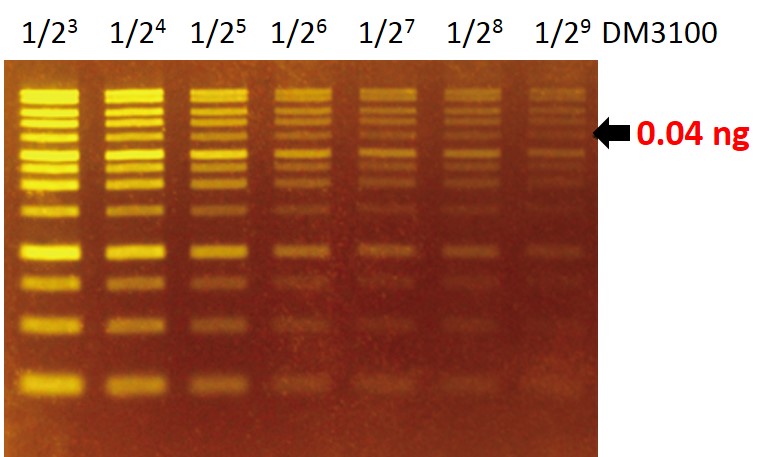
Sensitivity of FluoroStain™
The FluoroStain™ DNA Fluorescent Staining Dye (DS1000) shows a green-yellow fluorescence under blue light excitation. The sensitivity of DS1000 is about 0.04 ng (arrow) for a 4 kb fragment.
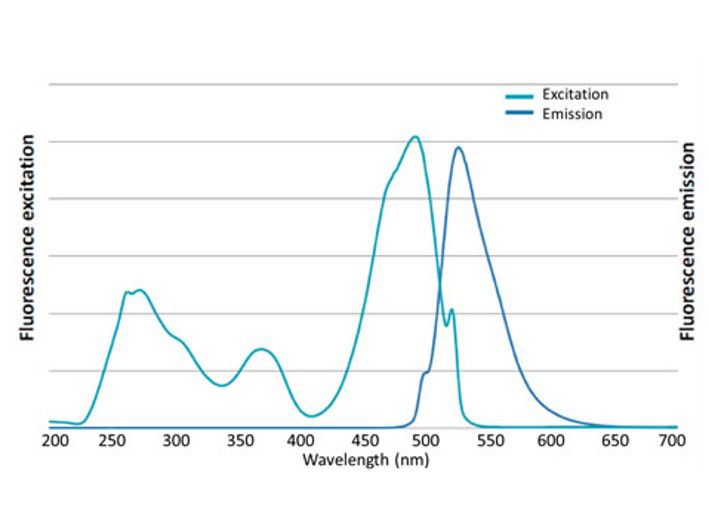
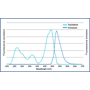
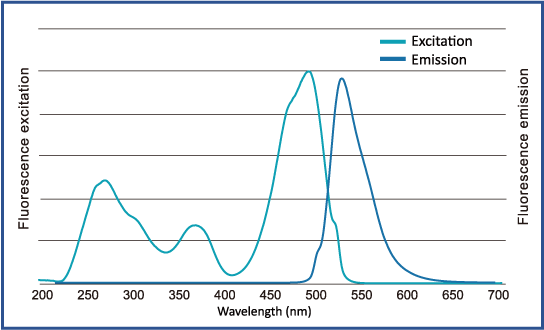
Excitation and emission spectrum of FluoroStain™
FluoroStain™ DNA Fluorescent Staining Dye (DS1000) emission as bound to dsDNA is 522 nm while its excitation peaks are at 270, 370 and 497 nm.
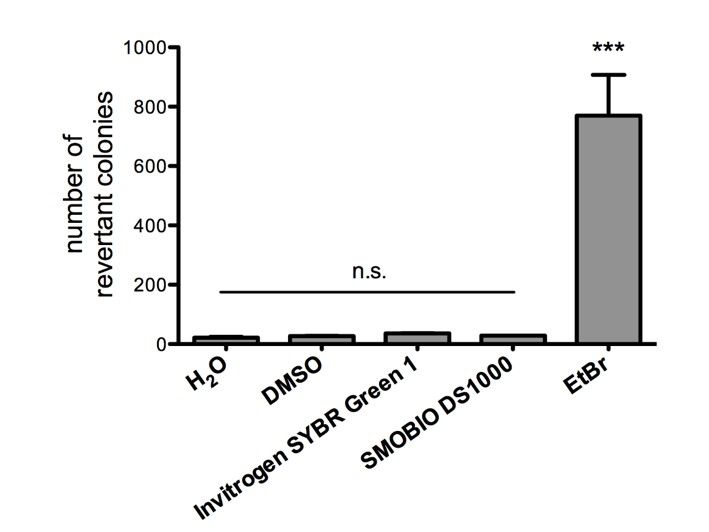
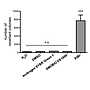
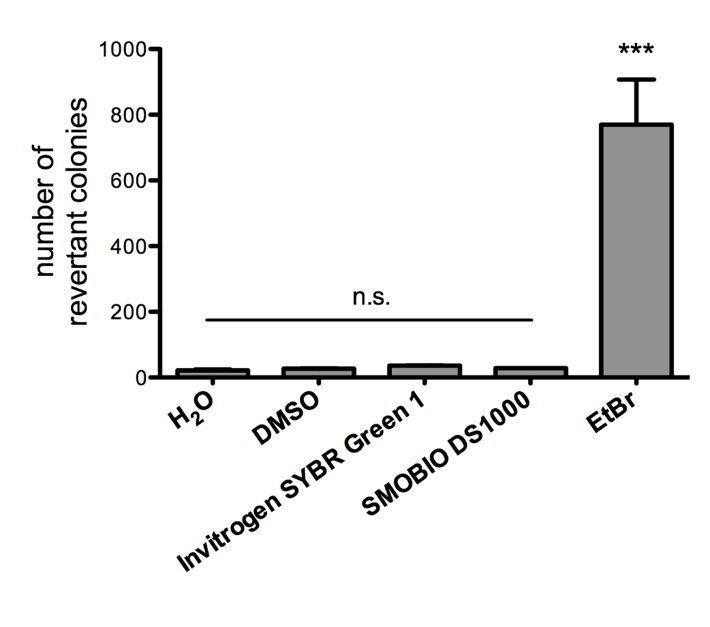
Non-mutagenicity of FluoroStain™
FluoroStain™ DNA Fluorescent Staining Dye (DS1000) is proofed for their safety (non-mutagenicity) using Ames test. However, it must be noted that since solvent may penetrate the skin, it is recommended that users wear gloves when using the fluorescent dyes.
Contents
|
Component |
Volume |
Cat. No. |
|
FluoroStain™ DNA Fluorescent Staining Dye (Green, 10,000X) |
500 μl |
DS1000 |
|
FluoroStain™ DNA Fluorescent Staining Dye (Green, 10,000X) |
500 μl x 5 |
DS1001 |
Storage
Protected from light
4°C for 12 months
-20°C for 24 months
Will FluoroStain™ DNA Fluorescent Staining Dye (DS1000) affect the DNA samples in subsequent experiments?
Using FluoroStain™ DNA Fluorescent Staining Dye (DS1000) does not affect subsequent operations. This is because the fluorescent dye can easily be removed by regular alcohol precipitation or gel elution kits.
Will the FluoroStain™ DNA Fluorescent Staining Dye (DS1000) affect cloning efficiency?
Using the fluorescent dye coupled with blue light, the cloning efficiency is increased by about 100 times compared to using EtBr and UV light.
Are there relevant documents that support safety of FluoroStain™ DNA Fluorescent Staining Dye (DS1000)?
FluoroStain™ DNA Fluorescent Staining Dye (DS1000) is proofed for their safety (non-mutagenicity) using Ames test (Figures below). However, it must be noted that since solvent may penetrate the skin, it is recommended that users wear gloves when using the fluorescent dyes.
Is it possible to use FluoroStain™ DNA Fluorescent Staining Dye (DS1000) instead of ethidium bromide (EtBr) in staining of DGGE gels which is composed of poly acrylamide?
In the case of DGGE staining with FluoroStain™ DNA Fluorescent Staining Dye (DS1000), we can give some suggestions as stated below:
A. Similar to ethidium bromide, DS1000 could be used for staining DNA in polyacrylamide gels.
B. However, it is noted that the DS1000 is designed to stain dsDNA and thus is not appropriate for staining in DGGE due to denaturing of dsDNA.
C. For detection of ssDNA or RNA in DGGE, we strongly recommend the use of FluoroVue™ Nucleic Acid Gel Stain (NS1000) which can be used to stain both double-strands and single-strand nucleic acids.
D. Furthermore, it’s suggested staining the gel after electrophoresis (post-staining) instead of pre-staining or in-gel staining, since the fluorescent dye might be affected during acrylamide polymerization.
E. The optimal excitation wavelength of DS1000 or NS1000 is around 480~490 nm (blue light), and therefore the illuminator with blue light is preferred. Of course, UV could be used also, but the signals detected might be weaker.
In using FluoroStain™ DNA Fluorescent Staining Dye (DS1000), which method is recommended as the gel soaking and in-gel staining methods (pre-casted gel with fluorescent dye) are taken into account?
FluoroStain™ DNA Fluorescent Staining Dye (DS1000) should be used by gel soaking (stain the gel after electrophoresis). For in-gel stain, FluoroVue™ Nucleic Acid Gel Stain (NS1000) is suggested.
After post staining using the FluoroStain™ DNA Fluorescent Staining Dye (DS1000), why the DNA bands are sometimes blurred up and the fluorescent signal is not enough?
There are three possible reasons:
A. The fluorescent dye is very sensitive, so a small amount of smear due to DNA quality may blur the band.
B. The agarose is impure, or incompletely dissolved. Tailing effect may also cause the fluorescent signal to weaken.
C. There may not be enough dye or the staining time is insufficient. After each staining, gels will absorb a large amount of fluorescent dye, thus causing insufficient staining for the next time if the same staining bath is repeatedly used. Furthermore, the time needed for staining can be elongated based on the percentage and the thickness of the agarose gel; harder and thicker gels require a longer staining period.
Do you recommend using DNA staining dye diluted in loading buffer?
We do not recommend this because the wrong concentration after dilution may cause inconsistent results.
Does the quality of agarose gel matter when using FluoroStain™ DNA fluorescent staining dye (DS1000)?
Yes, insufficient quality of agarose gel may lead to the limited performance of the dye.
What is the generally recommended dilution of FluoroStain™ DNA fluorescent staining dye (DS1000)?
A dilution of 10,000 times. For example, 10 μL staining dye is added to 100 mL of water, TE, TAE, or TBE. Next soak the agar gel in the solution. Soaking time depends on the percentage and thickness of agarose gel.
Does using FluoroStain™ DNA Fluorescent Staining Dye (DS1000) for staining the DNA cause bias in DNA molecular weight determination?
No, when the gel is stained with FluoroStain™ DNA staining dye (DS1000) after electrophoresis, the DNA molecular weight interpretation is accurate.
How many ways can the FluoroStain™ DNA Fluorescent Staining Dye (DS1000) be used?
For using the FluoroStain™ DNA Fluorescent Staining Dye (DS1000), it is recommended to be used with post staining method. If DS1000 is used with in-gel staining or staining during electrophoresis, the DNA band may not be clear enough and DNA migration may be changed.
Why does the gel stained with FluoroStain™ DNA Fluorescent Staining Dye (DS1000) show minimal background which is barely seen as compared with DNA signals that are strongly fluorescent after staining?
FluoroStain™ DNA Fluorescent Staining Dye (DS1000) manifests fluorescent signal when it properly binds with double stranded DNA.
Can FluoroStain™ DNA Fluorescent Staining Dye (DS1000) be used to observe RNA?
FluoroStain™ DNA Fluorescent Staining Dye (DS1000) is designed specifically for double strand DNA staining not for RNA use. If there is the need to stain RNA, it is recommended to use FluoroVue™ nucleic acid stain (NS1000) to stain RNA.
During the dilution of FluoroStain™ DNA Fluorescent Staining Dye (DS1000), it‘s occasionally seen that there are few red flocculent unable to be completely dissolved in the buffer. Is there any method to improve this?
To avoid the solid precipitation of FluoroStain™ DNA Fluorescent Staining Dye (DS1000) during -20℃ (4℃) storage, we recommend opening the storage tubes only when the DS1000 solution is complete melted.
If solid precipitation of DS1000 was observed, warm the tube at 37℃ for 10 min and then vortex to dissolve the solid particles. If the solid precipitation of DS1000 was still unable to be completely dissolved, spin down the precipitate and use only supernatant to dilute in TAE/TBE/TE buffer. Few solid precipitation of DS1000 will not cause the decline of sensitivity.
FluoroStain™ DNA Fluorescent Staining Dye (DS1000) Retention period: Mentioned -20℃ and over 2 years. Is it possible to have a retention period of 30 months or 36 months? Have you tested this period?
In our tests, the retention period of FluoroStain™ DNA Fluorescent Staining Dye (DS1000) could extend beyond 2 years if it is maintained at the recommended temperature of -20℃. However, we must recommend that the retention period should still be 2 years. Storage conditions should be in a dark room and temperature kept at -20℃. High temperature and light will result in fluorescent dye attenuation. We have tested our DS1000 at -20℃ for 30 months and it did perform with similar efficiency. Again, we do not recommend a retention period of more than 24 months at -20℃.
How many cycles of freezing and thawing can the FluoroStain™ DNA Fluorescent Staining Dye (DS1000) handle? If 5 μL is used at once, will it have a usage of 100 times?
Our tests have shown that 100 freezing and thawing cycles will be appropriate for the FluoroStain™ DNA Fluorescent Staining Dye (DS1000). In DS1000's usage information, it is indicated that the fluorescent dye should be protected from light and kept at low temperatures. After using DS1000, it should immediately be kept between 4℃ to -20℃ as fluorescent dye decays at a faster rate at room temperature. Please remember that any unthawed solution will cause the fluorescent dye concentration to be less after each use therefore reducing its sensitivity performance.
Is there any information on the ionic strength, pH stability, and optimum pH for FluoroStain™ DNA Fluorescent Staining Dye (DS1000)?
FluoroStain™ DNA Fluorescent Staining Dye (DS1000) stock is diluted in DMSO and does not have any special pH range. It is not advisable to use DS1000 in water. Therefore, it should be used with 1x TAE, TBE, and TE buffer at pH8.0 to gain the desired results. The optimum pH value for DS1000 is 7.5 to 8.0. Less than 7.5 and more than 8.0 may result in reduced sensitivity.
Can the FluoroStain™ DNA Fluorescent Staining Dye (DS1000) be removed from DNA? How can we do it?
The staining dye can be removed from DNA with traditional ethanol precipitation, PCR clean up kits, or the gel extraction kits. The ethanol precipitation method can follow conventional molecular cloning or follow the listed protocol. Ethanol precipitation.
A. Measure the volume of the DNA sample.
B. Add 1/10 volume of 3M sodium acetate, pH 5.2,(final concentration of 0.3 M) - The pH value of 3M sodium acetate must be adjusted with acetate not with HCl.
C. Mix well.
D. Add 2 to 2.5 volumes of cold 100% ethanol (calculated after salt addition).
E. Mix well.
F. Place on ice or at -20 °C for >20 minutes.
G. Spin at maximum speed in a microfuge for 10-15 min.
H. Carefully decant supernatant.
I. Add 1 mL 70% ethanol. Rinse and spin briefly. Carefully decant supernatant.
J. Air dry or briefly vacuum dry pellet.
K. Re-suspend pellet in the appropriate volume of TE, Tris buffer or water.
Can the FluoroStain™ DNA Fluorescent Staining Dye (DS1000) stained DNA be used for enzymatic reaction like ligation, enzyme digestion, PCR and so on? Do you have any recommended methods like pretreatment?
After removing the fluorescent staining dye from DNA, the DNA can be used for ligation, restriction enzyme digestion or PCR reaction. It is imperative that the DNA is maintained in good quality for bioassay after removing the stained dye. We recommend removing the fluorescent staining dye with the gel extraction kits or PCR clean up kits because these methods are convenient and high efficiency. The very low amount of DS1000 does not affect ligation, enzyme digestion or PCR. However, the threshold is related to the enzyme systems and it is on a case-by-case basis. For the best results, we recommend to remove the staining dye from DNA before proceeding to the next step of the experiment.
How do we dispose of the staining solution? Is the disposal method for EtBr solution acceptable for this product?
We recommend that disposal be made in accordance to the local law. The freshly prepared FluoroStain™ DNA Fluorescent Staining Dye (DS1000) solution can be filtered through activated charcoal before disposal. The charcoal can then be disposed of by incineration. One gram of activated charcoal easily absorbs the dye from 10 liters of freshly prepared working solution.
What is the stability of the FluoroStain™ DNA Fluorescent Staining Dye (DS1000)? Is it stable in hot condition and does it use in-gel or post staining?
FluoroStain™ DNA staining dye (DS1000) can be stored at -20℃ for at least 24 months. If it needs to be used frequently, it can be stored at room temperature or 4℃. The DS1000 is recommended to be used with post stain method. For in-gel staining the best choice would be to use FluoroVue™ Nucleic Acid Gel Stain (NS1000).
Before opening
Warm the vial to an ambient temperature until the solution is thawed thoroughly.
Vortex and spin down the content of the vial to ensure the solution is homogeneous.
Working Reagent Preparation
1:10,000 dilution in TE, TAE or TBE buffer
Staining after electrophoresis (post-staining)
This protocol is highly recommended.
1. Performing agarose gel electrophoresis following your standard protocol.
2.Dilute FluoroStain™ DNA Fluorescent Staining Dye reagent 10,000 folds in a TE, TAE, or TBE buffer.
For example: 10 μl in 100 ml TAE buffer
Use a plastic container. Glass containers are not recommended, as they absorb fluorescent dye in staining solution.
Protect the staining container from light by covering it with aluminium foil, or place it in the dark.
The staining solution can be stored for up to one week or more.
3. Immerse the gel in a staining solution (1X) and incubate at room temperature for 10 - 30 minutes. (avoid light).
Staining time varies with the thickness of the gel and percentage of agarose. If needed, agitate the gel gently at room temperature to shorten staining time.
De-staining is not required.
4. Visualize or photograph the gel with UV or blue-light illumination (blue-light is recommended).
Clean the surface of the illuminator before and after each use with deionized water. Accumulation of fluorescent dyes on the surface will create a high fluorescent background.
Video cameras and CCD cameras have a different spectral response compared to the black-and-white print film and therefore may not exhibit the same sensitivity.
Wang YZ, Shen CC, Cheng YC, Lin SM. Sci Rep. 2025 Oct 14;15(1):35909. doi: 10.1038/s41598-025-19878-8
PMCID: PMC12521355
A multiplex PCR method to determine the sex of fetal rat tissues
Cristine Camp, Paige Drotos, Adrian Courville, Miranda Reed, Rachel West
Mol Biol Rep. 2025 Mar 13;52(1):301. doi: 10.1007/s11033-025-10406-5
PMCID: PMC11906549
Description of a new music frog (Anura, Ranidae, Nidirana) critically endangered in Taiwan
Chun-Fu Lin, Chunwen Chang, Masafumi Matsui, Chin-Chia Shen, Atsushi Tominaga, Si-Min Lin
Zookeys. 2025 Feb 27:1229:245-273. doi: 10.3897/zookeys.1229.139344. eCollection 2025
PMCID: PMC11886882
Recombinant expression and characterization of Canine circovirus capsid protein for diagnosis
Wichan Dankaona, Pornpiroon Nooroong, Napassorn Poolsawat, Chutchai Piewbang, Somporn Techangamsuwan, Panat Anuracpreeda
Front Vet Sci. 2024 Apr 10:11:1363524. doi: 10.3389/fvets.2024.1363524. eCollection 2024
PMCID: PMC11040689
Ahmed Namisy, Shu-Yun Chen, Jin-Hsing Huang, Jintana Unartngam, Chinnapan Thanarut, Wen-Hsin Chung
Microbiol Spectr. 2024 Feb; 12(2): e03127-23. Published online 2024 Jan 4. doi: 10.1128/spectrum.03127-23
PMCID: PMC10846128
Napassorn Poolsawat, Siriphan Sangchuai, Tassanee Jaroensak, Amaya Watthanadirek-Wijidwong, Nitipon Srionrod, Sutthida Minsakorn, Keiichiro Tazawa, Panat Anuracpreeda
Sci Rep. 2023; 13: 20394. Published online 2023 Nov 21. doi: 10.1038/s41598-023-47784-4
PMCID: PMC10663595
Piyanun Harnpicharnchai, Panyapon Pumkaeo, Paopit Siriarchawatana, Somsak Likhitrattanapisal, Sermsiri Mayteeworakoon, Lily Ingsrisawang, Worawongsin Boonsin, Lily Eurwilaichitr, Supawadee Ingsriswang. PLoS One. 2023; 18(6): e0287567. Published online 2023 Jun 29. doi: 10.1371/journal.pone.0287567
PMCID: PMC10309600
Nitipon Srionrod, Pornpiroon Nooroong, Napassorn Poolsawat, Sutthida Minsakorn, Amaya Watthanadirek, Witchuta Junsiri, Siriphan Sangchuai, Runglawan Chawengkirttikul, and Panat Anuracpreeda
Front Cell Infect Microbiol. 2022; 12: 1065963. Published online 2022 Nov 29. doi: 10.3389/ fcimb.2022.1065963
PMCID: PMC9744959
Sho Sugawara, Ryo Okada, Tze Mun Loo, Hisamichi Tanaka, Kenichi Miyata, Masatomo Chiba, Hiroko Kawasaki, Kaoru Katoh, Shizuo Kaji, Yoshiro Maezawa, Koutaro Yokote, Mizuho Nakayama, Masanobu Oshima, Koji Nagao, Chikashi Obuse, Satoshi Nagayama, Keiyo Takubo, Akira Nakanishi, Masato T. Kanemaki, Eiji Hara, Akiko Takahashi
Commun Biol. 2022; 5: 1420. Published online 2022 Dec 28. doi: 10.1038/ s42003-022-04369-7
PMCID: PMC9797495
Keiichiro Tazawa, Napassorn Poolsawat, Andrew D Gibson, Luke Gamble, Alasdair King, Panat Anuracpreeda, Vet World. 2022 Apr;15(4):1121-1128. doi: 10.14202/vetworld.2022.1121-1128. Epub 2022 Apr 28.
PMCID: PMC9178580
Molecular detection and genetic diversity of Leucocytozoon sabrazesi in chickens in Thailand
Runglawan Chawengkirttikul, Witchuta Junsiri, Amaya Watthanadirek, Napassorn Poolsawat, Sutthida Minsakorn, Nitipon Srionrod, Panat Anuracpreed, Sci Rep. 2021 Aug 17;11(1):16686. doi: 10.1038/s41598-021-96241-7.
PMCID: PMC8370975

B-BOX™ Blue Light LED Epi-illuminator
470 nm long wavelength
Improved cloning efficiency
Compact, light-weight, and portable (less than 1 kg)
Adjustable and removable filter plate allows for gel cutting, visualization, and documentation
470 nm long wavelength
Improved cloning efficiency
Compact, light-weight, and portable (less than 1 kg)
Adjustable and removable filter plate allows for gel cutting, visualization, and documentation
ExcelBand™ DNA Ladder series
Sharp bands
Quick reference— enhanced bands
Ready-to-use— premixed with loading dye for direct loading
Stable— room temperature storage over 6 months

FluoroVue™ Nucleic Acid Gel Stain
Excellent for in-gel staining
Sensitivity up to 0.14 ng (DNA) or or 1 ng (total RNA)
A safe alternative to EtBr
Suitable for blue or UV light

![[DS1000] FluoroStain™ DNA Fluorescent Staining Dye (Green, 10,000X), 500 μl](/web/image/product.template/334/image?unique=9adbb93)
![[DS1000] FluoroStain™ DNA Fluorescent Staining Dye (Green, 10,000X), 500 μl](/website/image/product.template/334/image/90x90)