Description
FluoroVue™ Nucleic Acid Gel Stain (10,000X) is specially designed for in-gel use and is a safer replacement for conventional Ethidium bromide (EtBr), which poses a significant health and safety hazard to its users. It is a fluorescent stain which offers highly sensitive detection of double-stranded or single-stranded DNA and RNA in a convenient manner. FluoroVue™ Nucleic Acid Gel Stain offers high sensitivity that is several times greater than EtBr.
FluoroVue™ Nucleic Acid Gel Stain is compatible with both conventional UV gel-illumination systems as well as harmless long wavelength blue light illumination systems, like B-BOX™. When bound to nucleic acids, FluoroVue™ Nucleic Acid Gel Stain has a fluorescent excitation maximum of 250 and 482 nm, and an emission maximum of 509 nm. Therefore, it can replace EtBr without the need of changing existing lab imaging systems.
Features:
Excellent for in-gel staining
Sensitivity: 0.14 ng (DNA) or 1 ng (total RNA)
A safer alternative to EtBr
Compatibility: suitable to blue or UV light
Increased cloning efficiency (blue light)
Storage
Protected from light
4°C for 12 months
-20°C for 24 months
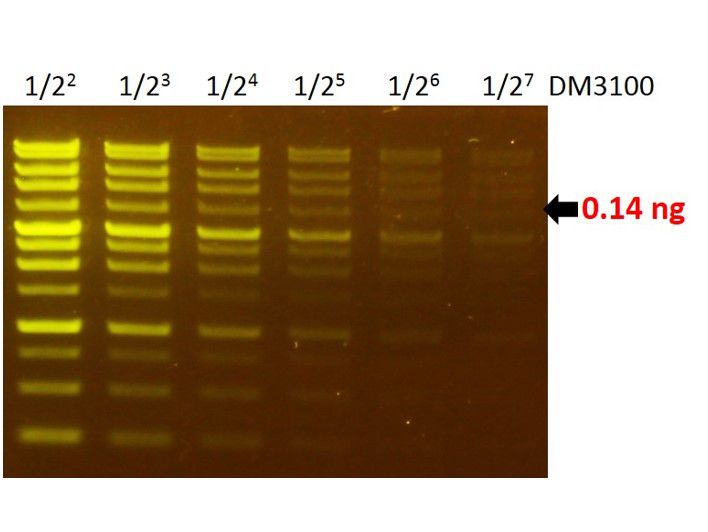
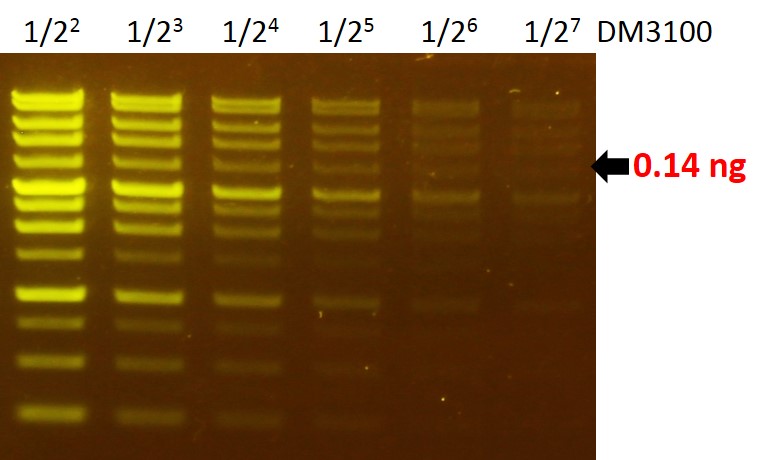
Sensitivity of FluoroVue™
The FluoroVue™ Nucleic Acid Gel Stain (NS1000) shows a green-yellow fluorescence under blue light excitation. The sensitivity of NS1000 is about 0.14 ng (arrow) for a 4 kb fragment.
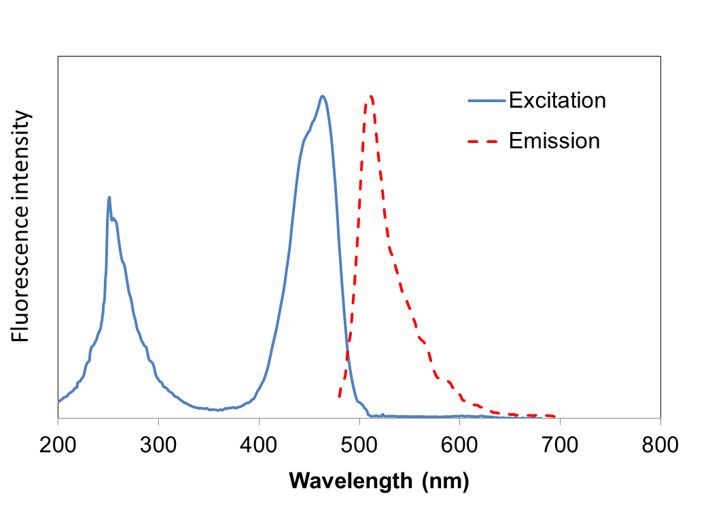
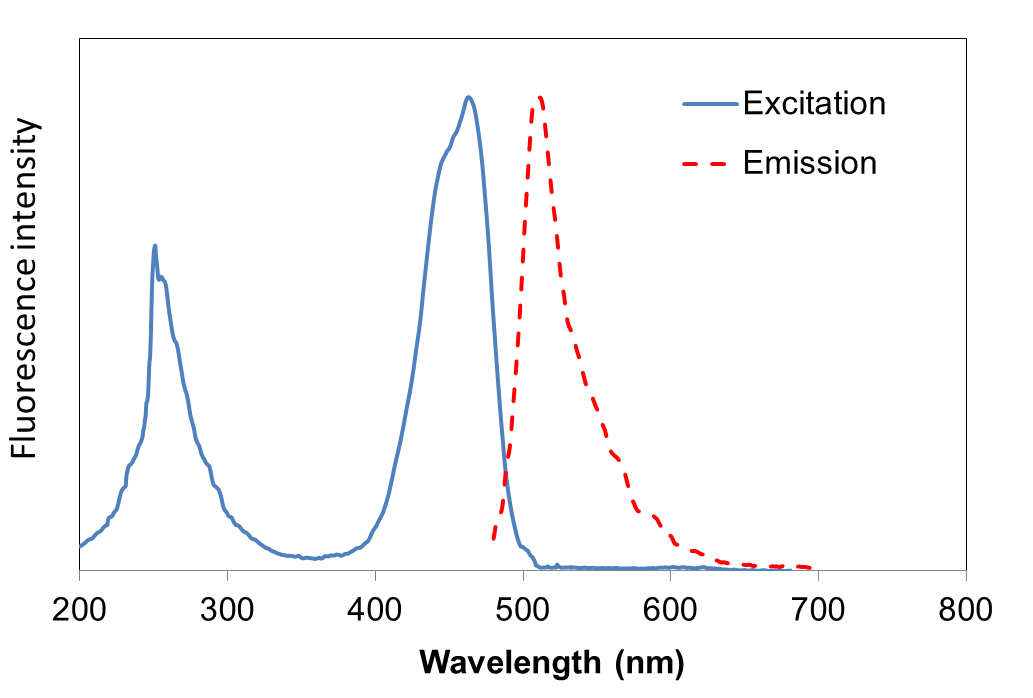
Excitation and emission spectrum of FluoroVue™
FluoroVue™ Nucleic Acid Gel Stain (NS1000) has a fluorescent excitation maxima of ~250 and ~482 nm, and an emission maximum of ~509 nm. Therefore, it can replace EtBr without the need for changing existing lab imaging systems.
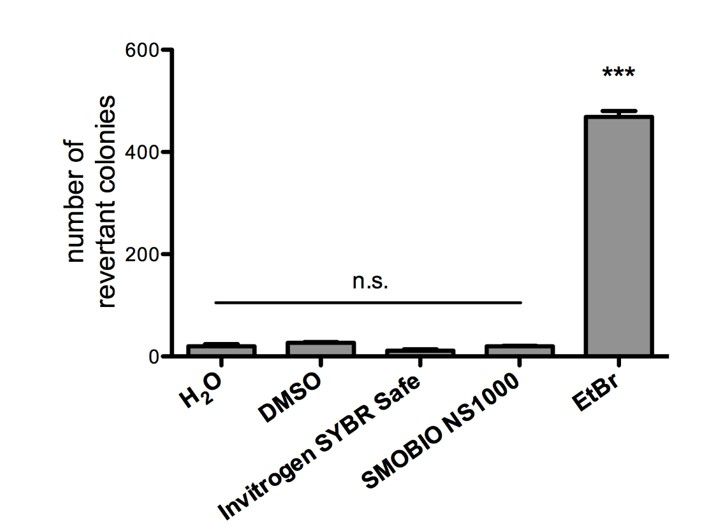
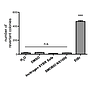
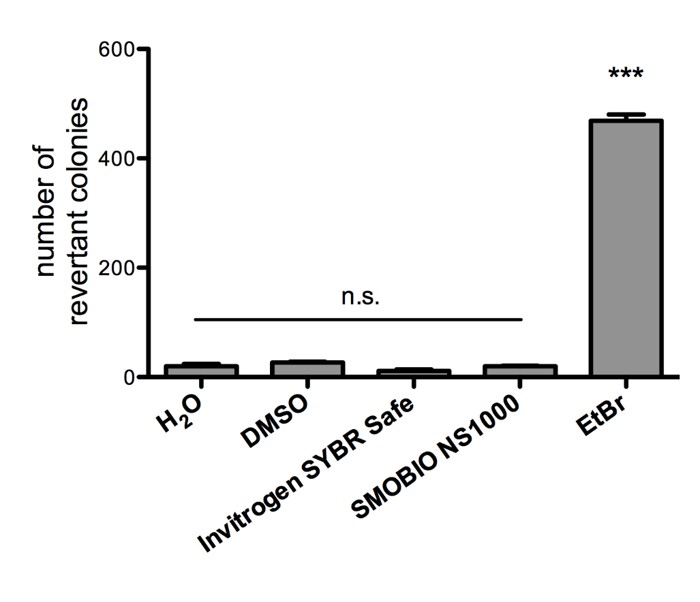
Non-mutagenicity of FluoroVue™
FluoroVue™ Nucleic Acid Gel Stain (NS1000) is proofed for their safety (non-mutagenicity) using Ames test. However, it must be noted that since solvent may penetrate the skin, it is recommended that users wear gloves when using the fluorescent dyes.
Contents
Component | Volume | Cat. No. |
FluoroVue™ Nucleic Acid Gel Stain (10,000X) | 500 μl | NS1000 |
| FluoroVue™ Nucleic Acid Gel Stain (10,000X) | 5 x 500 μl | NS1001 |
Storage
Protected from light
4°C for 12 months
-20°C for 24 months
Will FluoroVue™ Nucleic Acid Gel Stain (NS1000) affect the DNA/RNA samples in subsequent experiments?
Using FluoroVue™ Nucleic Acid Gel Stain (NS1000) does not affect subsequent operations. This is because the fluorescent dye can easily be removed by regular alcohol precipitation or gel elution kits.
Will the FluoroVue™ Nucleic Acid Gel Stain (NS1000) affect cloning efficiency?
Using the fluorescent dye coupled with blue light, the cloning efficiency is increased by about 100 times compared to using EtBr and UV light.
Are there relevant document that support safety of FluoroVue™ Nucleic Acid Gel Stain (NS1000)?
FluoroVue™ Nucleic Acid Gel Stain (NS1000) is proofed for their safety, including non-mutagenicity and non-cytotoxicity (documents below). However, it must be noted that since solvent may penetrate the skin, it is recommended that users wear gloves when using the fluorescent dyes.
Does the quality of agarose gel matter when using FluoroVue™ Nucleic Acid Gel Stain (NS1000)?
Yes, insufficient quality of agarose gel may lead to the limited performance of the dye.
How many ways can the FluoroVue™ Nucleic Acid Gel Stain (NS1000) be used? ?
For using the FluoroVue™ Nucleic Acid Gel Stain (NS1000), it is highly recommended to be used with in-gel staining method. NS1000 can also be used with post staining or staining during electrophoresis.
Why does the gel stained with FluoroVue™ Nucleic Acid Gel Stain (NS1000) show minimal background which is barely seen as compared with DNA/RNA signals that are strongly fluorescent after staining?
The FluoroVue™ Nucleic Acid Gel Stain (NS1000) exhibits fluorescent signal when it binds with nucleic acid (dsDNA, ssDNA, and RNA).
How many cycles of freezing and thawing can the FluoroVue™ Nucleic Acid Gel Stain (NS1000) handle? If 5 μL is used at once, will it have a usage of 100 times?
Our tests have shown that 100 freezing and thawing cycles will be appropriate for the FluoroVue™ Nucleic Acid Gel Stain (NS1000). In NS1000's usage information, it is indicated that the fluorescent dye should be protected from light and kept at low temperatures. After using NS1000, it should immediately be kept between 4℃ to -20℃ as fluorescent dye decays at a faster rate at room temperature. Please remember that any unthawed solution will cause the fluorescent dye concentration to be less after each use therefore reducing its sensitivity performance.
Can the FluoroVue™ Nucleic Acid Gel Stain (NS1000) be removed from DNA? How can we do it?
The staining dye can be removed from DNA with traditional ethanol precipitation, PCR clean up kits, or the gel extraction kits. The ethanol precipitation method can follow conventional molecular cloning or follow the listed protocol. Ethanol precipitation.
A. Measure the volume of the DNA sample.
B. Add 1/10 volume of 3M sodium acetate, pH 5.2,(final concentration of 0.3 M) - The pH value of 3M sodium acetate must be adjusted with acetate not with HCl.
C. Mix well.
D. Add 2 to 2.5 volumes of cold 100% ethanol (calculated after salt addition).
E. Mix well.
F. Place on ice or at -20 °C for >20 minutes.
G. Spin at maximum speed in a microfuge for 10-15 min.
H. Carefully decant supernatant.
I. Add 1 mL 70% ethanol. Rinse and spin briefly. Carefully decant supernatant.
J. Air dry or briefly vacuum dry pellet.
K. Re-suspend pellet in the appropriate volume of TE, Tris buffer or water.
Can the FluoroVue™ Nucleic Acid Gel Stain (NS1000) stained DNA be used for enzymatic reaction like ligation, enzyme digestion, PCR and so on? Do you have any recommended methods like pretreatment?
After removing the fluorescent staining dye from DNA, the DNA can be used for ligation, restriction enzyme digestion or PCR reaction. It is imperative that the DNA is maintained in good quality for bioassay after removing thefluorescent staining dye. We recommend removing the FluoroVue™ Nucleic Acid Gel Stain (NS1000) with the gel extraction kits or PCR clean up kits because these methods are convenient and high efficiency. The very low amount of fluorescent staining dye does not affect ligation, enzyme digestion or PCR. However, the threshold is related to the enzyme systems and it is on a case-by-case basis. For the best results, we recommend to remove the staining dye from DNA before proceeding to the next step of the experiment.
How do we dispose of the staining solution? Is the disposal method for EtBr solution acceptable for this product?
We recommend that disposal be made in accordance to the local law. The freshly prepared FluoroVue™ Nucleic Acid Gel Stain (NS1000) solution can be filtered through activated charcoal before disposal. The charcoal can then be disposed of by incineration. One gram of activated charcoal easily absorbs the dye from 10 liters of freshly prepared working solution.
When the FluoroVue™ Nucleic Acid Gel Stain (NS1000) or EtBr is used for in-gel staining, why do some of the lowest bands become weaker in a longer agarose gel?
This is because EtBr or the dye component of FluoroVue™ Nucleic Acid Gel Stain (NS1000) keep moving toward the cathode in a direction opposite to the DNA migration. Therefore after electrophoresis the concentration of NS1000 or EtBr is very low near the anode end (the bottom) of the agarose gel, and some bands close to the bottom will be very weak in signal or even undetectable. It is recommend to reduce the electrophoresis time or use post staining method to improve the intensity of small fragments of DNA.
How do we deal with certain precipitate in NS1000 ?
Occasionally, some precipitate will be observed in NS1000 due to high concentration of dye ingredient.
If slight precipitate exists in NS1000, we suggest incubating the NS1000 at 37 ℃ for one hour until the precipitate is fully dissolved.
The sensitivity of NS1000 still keeps good after the precipitate is dissolved.
Li B, Zhai JQ, Wu YJ, Shan F, Zou JJ, Hou FH, Que TC, Chen W.
PLoS Negl Trop Dis. 2024 Nov 22;18(11):e0012667. doi: 10.1371/journal.pntd.0012667. eCollection 2024 Nov.
PMC11623466
Kwanjeera Wanichthanarak, Intawat Nookaew, Phongthana Pasookhush, Thidathip Wongsurawat, Piroon Jenjaroenpun, Namkhang Leeratsuwan, Songsak Wattanachaisaereekul, Wonnop Visessanguan, Yongyut Sirivatanauksorn, Narong Nuntasaen, Chutima Kuhakarn, Vichai Reutrakul, Pravech Ajawatanawong, Sakda Khoomrung
BMC Plant Biol. 2023 Jan 28;23(1):59. doi: 10.1186/s12870-023-04074-5.
PMCID: PMC9883906
Dwita Anastasia Deo, Elizabeth Henny Herningtyas, Umi Solekhah Intansari, Taufik Mulya Perdana, Elsa Herdiana Murhandarwati, Marsetyawan H N E Soesatyo, Trop Med Infect Dis. 2022 Jul 29;7(8):153. doi: 10.3390/tropicalmed7080153.
PMCID: PMC9412636
Dewa Gede Wiryangga Selangga, Listihani Listihani, HAYATI Journal of Biosciences, November 2022; 29(6), 806-813. https://doi.org/10.4308/hjb.29.6.806-813
Mina Rastgou, Vahid Roumi, Emanuela Noris, Slavica Matić, Sezai Ercisli, Plants (Basel). 2022 Apr 20;11(9):1118. doi: 10.3390/plants11091118.
PMCID: PMC9104223
Jessica Gasparello, Chiara Papi, Matteo Zurlo, Lucia Carmela Cosenza, Giulia Breveglieri, Cristina Zuccato, Roberto Gambari, Alessia Finotti, PLoS One. 2022 Apr 6;17(4):e0266419. doi: 10.1371/journal.pone.0266419. eCollection 2022.
PMCID: PMC8985952
Ocular surface toll like receptors in ageing
Antonio Di Zazzo, Maria De Piano, Marco Coassin, Tommaso Mori, Bijorn Omar Balzamino, Alessandra Micera, BMC Ophthalmol. 2022 Apr 22;22(1):185. doi: 10.1186/s12886-022-02398-8.
PMCID: PMC9027701
Distribution and molecular characterization of Squash mosaic virus on cucumber in Gianyar, Bali
Listihani, Ni Putu Pandawani, Tri Asmira Damayanti, Mimi Sutrawati, Dewa Gede Wiryangga Selangga, Ketut Ayu Yuliadhi, Trisna Agung Phabiola, & Gusti Ngurah Alit Susanta Wirya, J. Trop. Plant Pests Dis. Vol. 22, No. 1, March 2022: 48 –54
Seow-Chin Ong, Wei-Hung Cheng, Fu-Man Ku, Chih-Yu Tsai, Po-Jung Huang, Chi-Ching Lee 4, Yuan-Ming Yeh, Petr Rada, Ivan Hrdý, Ravi Kumar Narayanasamy, Tamara Smutná, Rose Lin, Hong-Wei Luo, Cheng-Hsun Chiu, Jan Tachezy, Petrus Tang, Genes (Basel). 2022 Mar 17;13(3):531. doi: 10.3390/genes13030531.
PMCID: PMC8951798
Association Between Retinoid X Receptor Gene Variants and Dyslipidemia Risk in an Iranian Population
Farzane Vafaeie, Toba Kazemi, Saeede Khosravi, Ebrahim Miri Moghaddam, Cureus. 2021 Sep 5;13(9):e17730. doi: 10.7759/cureus.17730. eCollection 2021 Sep.
PMCID: PMC8491560
Agus Setiawan, Wisnu Nurcahyo, Dwi Priyowidodo, Rina Tri Budiati, and Desy Sylvia Ratna Susanti, Vet World. 2021 Jan; 14(1): 113–119. Published online 2021 Jan 15. doi: 10.14202/vetworld.2021.113-119
PMCID: PMC7896907
PCR analysis for assessment of bacterial bioburden in orthokeratology lens case
Jung Lo, Po-Chiung Fang, Chun-Chih Chien, Chang-Chun Hsiao, Shin-Ling Tseng, Yu-Hsuan Lai, and Ming-Tse Kuocorresponding author, Mol Vis. 2016; 22: 1–8. Published online 2016 Jan 14
PMCID: PMC4734148
Yu-Ting Hsiao, Ming-Tse Kuo, Wei-Yu Chiang, Tsai-Ling Chao, and Hsi-Kung Kuo, BMC Ophthalmol. 2019; 19: 87. Published online 2019 Apr 3. doi: 10.1186/s12886-019-1093-2
PMCID: PMC6448235
Before opening
Warm the vial to an ambient temperature, then vortex and spin down the content of the vial to ensure the solution is homogeneous.
Working Reagent Preparation
1:10,000 dilution in TAE or TBE buffered agarose

In- gel staining
This protocol is highly recommended.
1. Prepare TAE or TBE buffered molten agarose solution.
40 ml molten agarose solution can cast two mini-gels (5.4 x 5.9 cm) or one landscape-gel (10.9 x 5.9 cm).
2. Dilute FluoroVue™ Nucleic Acid Gel Stain 10,000X with the molten gel solution and mix well prior to being poured into the gel.
For example: 4 μl in 40 ml molten agarose solution
3. Cool the molten agarose solution until it can be handled by hand and then pour it into gel tray.
Casted gels are stable at 4°C for 3 days in dark. After three days the sensitivity will decrease daily.
4. Perform agarose gel electrophoresis (avoid light).
The recommended voltage is 4–10 V/cm (distance between anode and cathode). Avoid using high voltage during electrophoresis. High voltage causes excess heat and affects the dye adversely.
During electrophoresis, the staining dye runs toward the cathode, therefore DNA bands with smaller molecular weights may be weaker in intensity due to less staining dye.
5. Visualize or photograph the gel with UV or blue-light illumination (blue-light is recommended).
Clean the surface of the illuminator before and after each use with deionized water. Accumulation of fluorescent dyes on the surface will create a high fluorescent background.
Video cameras and CCD cameras have a different spectral response compared to the black-and-white print film and therefore may not exhibit the same sensitivity.

Staining during electrophoresis
The sensitivity of this method is slightly lower than the in-gel staining.
1. Prepare agarose gel following your standard protocol.
2. Dilute FluoroVue™ Nucleic Acid Gel Stain 10,000 folds into the running buffer and mix well.
For example: 30 μl in 300 ml running buffer
3. Perform agarose gel electrophoresis (avoid light).
During electrophoresis, the staining dye runs toward the cathode .
4. Visualize or photograph the gel with UV or blue-light illumination (blue-light is recommended).
Clean the surface of the illuminator before and after each use with deionized water. Accumulation of fluorescent dyes on the surface will create a high fluorescent background.
Video cameras and CCD cameras have a different spectral response compared to the black-and-white print film and therefore may not exhibit the same sensitivity.
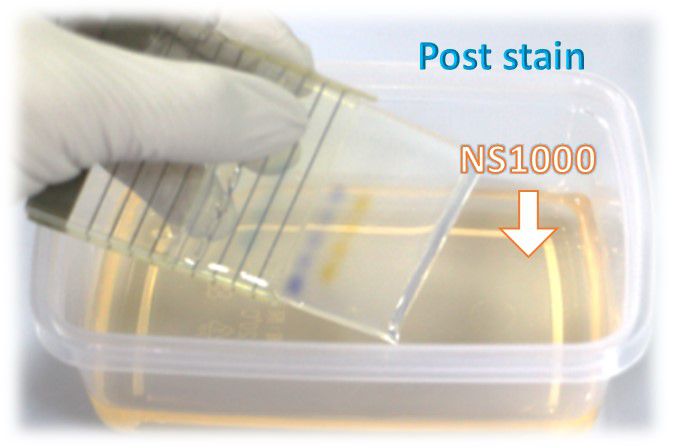
Staining after electrophoresis (post-staining)
Post-staining method is recommended for polyacrylamide electrophoresis, due to the longer time required for running PAGE. The sensitivity of this method is lower than the in-gel staining method.
1. Performing agarose gel electrophoresis following your standard protocol.
2. Dilute FluoroVue™ Nucleic Acid Gel Stain 10,000 folds into the TE, TAE, or TBE buffer and mix well.
For example: 10 μl in 100 ml TAE buffer
Use a plastic container. Glass containers are not recommended, as they absorb fluorescent dye in staining solution.
Protect the staining container from light by covering it with aluminium foil, or place it in the dark.
The staining solution can be stored for up to one week or more.
3. Immerse the gel in a staining solution (1X) and incubate at room temperature for 10 - 30 minutes. (avoid light).
Staining time varies with the thickness of the gel and percentage of agarose. If needed, agitate the gel gently at room temperature to shorten staining time.
4. Visualize or photograph the gel with UV or blue-light illumination (blue-light is recommended).
Clean the surface of the illuminator before and after each use with deionized water. Accumulation of fluorescent dyes on the surface will create a high fluorescent background.
Video cameras and CCD cameras have a different spectral response compared to the black-and-white print film and therefore may not exhibit the same sensitivity.

B-BOX™ Blue Light LED Epi-illuminator
470 nm long wavelength
Improved cloning efficiency
Compact, light-weight, and portable (less than 1 kg)
Adjustable and removable filter plate allows for gel cutting, visualization, and documentation
470 nm long wavelength
Improved cloning efficiency
Compact, light-weight, and portable (less than 1 kg)
Adjustable and removable filter plate allows for gel cutting, visualization, and documentation
ExcelBand™ DNA Ladder series
Sharp bands
Quick reference— enhanced bands
Ready-to-use— premixed with loading dye for direct loading
Stable— room temperature storage over 6 months

FluoroStain™ DNA Fluorescent Staining Dye
Excellent for post staining
Sensitivity up to 0.04 ng DNA
A safe alternative to EtBr
Suitable for blue or UV light

![[NS1000] FluoroVue™ Nucleic Acid Gel Stain (10,000X), 500 μl](/web/image/product.template/342/image?unique=49135dc)
![[NS1000] FluoroVue™ Nucleic Acid Gel Stain (10,000X), 500 μl](/website/image/product.template/342/image/90x90)