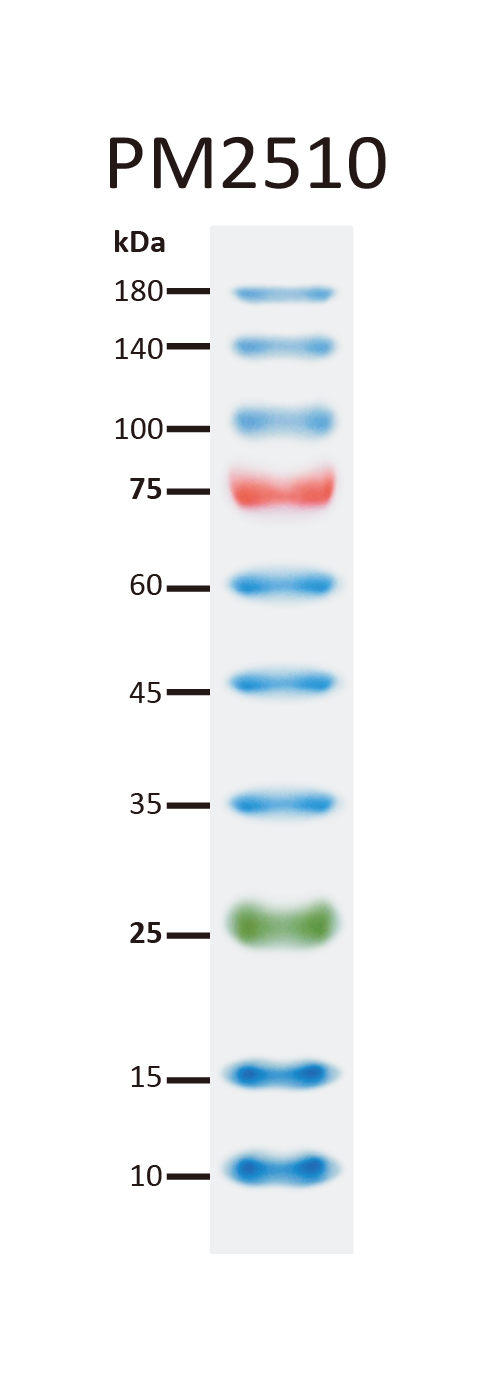
[PM2510] ExcelBand™ Enhanced 3-color Regular Range Protein Marker (9-180 kDa), 250 μl x 2
Description
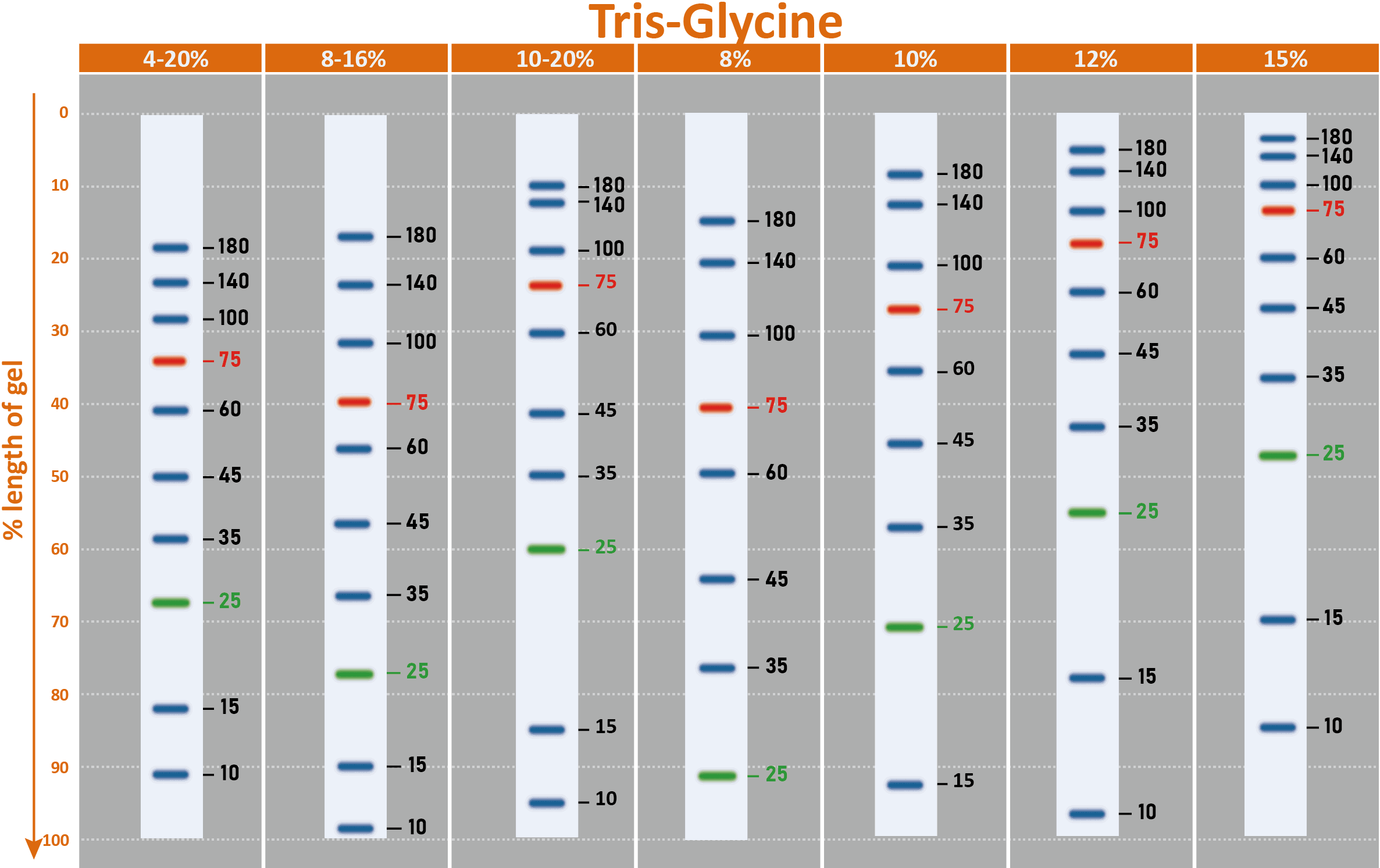
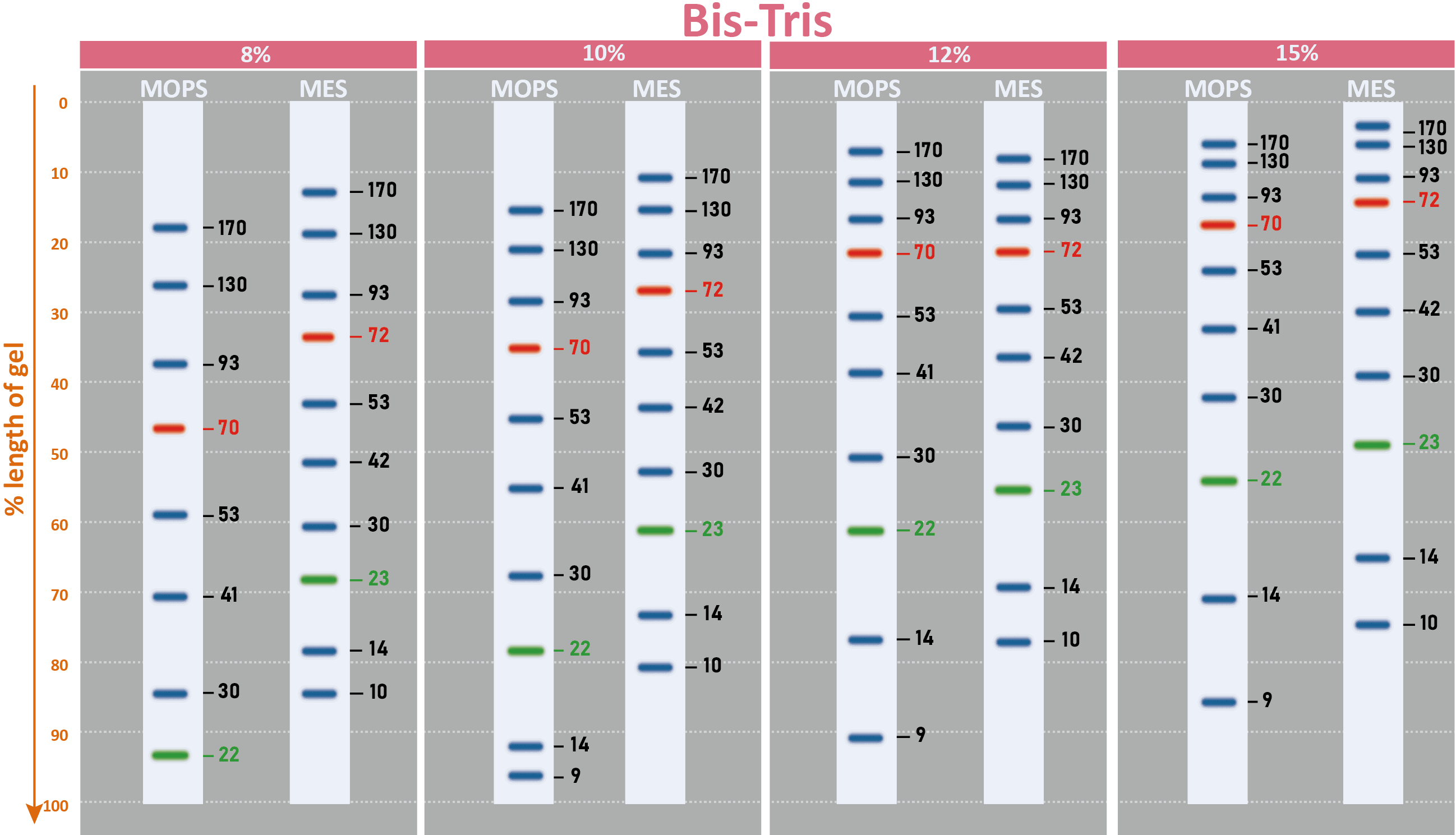
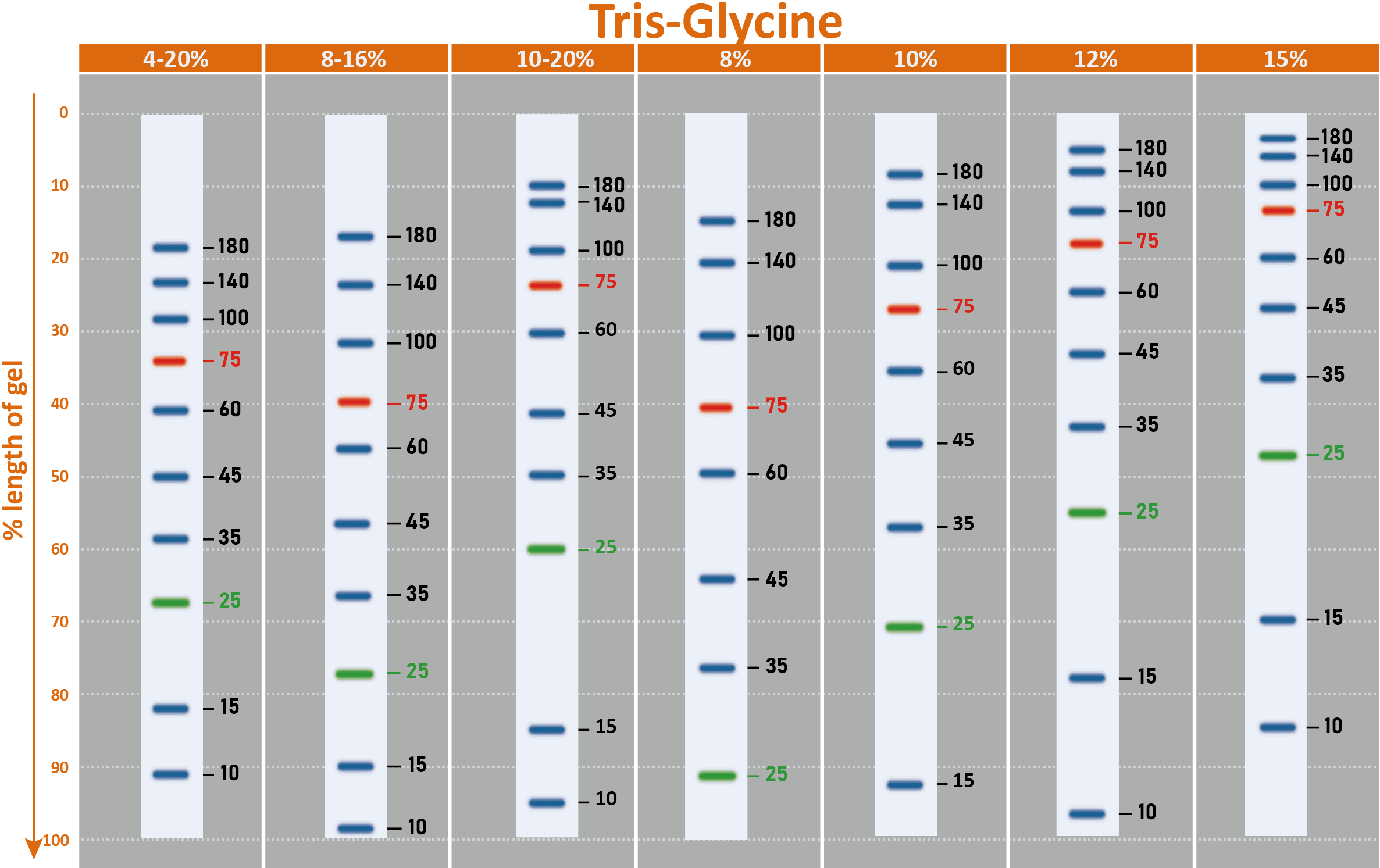
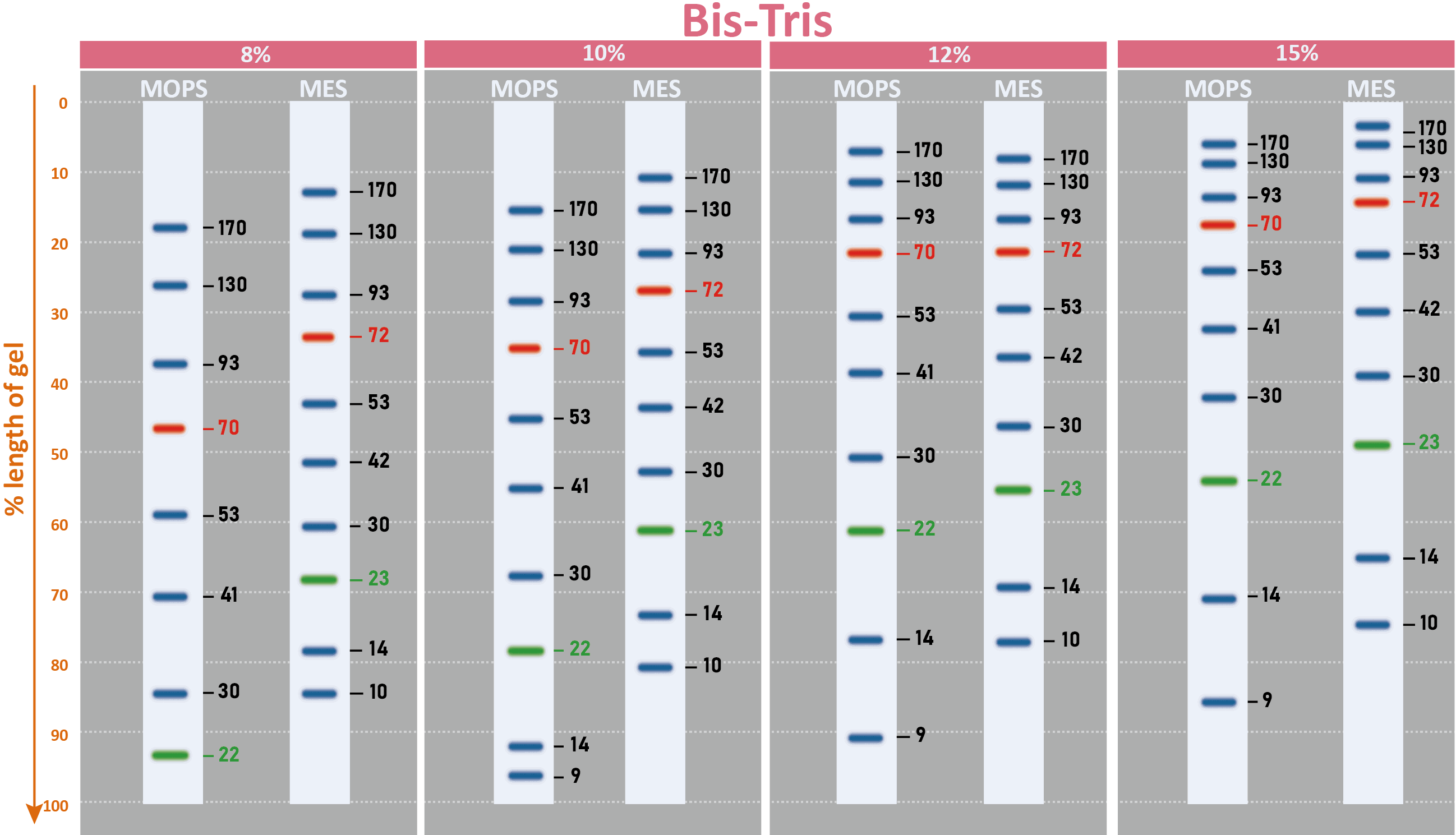
The PM2510 ExcelBand™ Enhanced 3-color Regular Range Protein Marker is a ready-to-use three-color protein standard with 10 pre-stained proteins covering a wide range of molecular weights from 10 to 180 kDa in Tris-Glycine Buffer (9 to 170 kDa in Bis-Tris (MOPS) buffer and 10 to 170 kDa Bis-Tris (MES) buffer). Proteins are covalently coupled with a blue chromophore except for two reference bands (one green and one red band at 25 kDa and 75 kDa respectively) when separated on SDS-PAGE (Tris-Glycine buffer). PM2510 ExcelBand™ Enhanced 3-color Regular Range Protein Marker is designed for monitoring protein separation during SDS-polyacrylamide gel electrophoresis, verification of Western transfer efficiency on membranes (PVDF, nylon, or nitrocellulose) and for approximating the size of proteins.
Features
Ready-to-use — Premixed with a loading buffer for direct loading, no need to boil.
Two reference bands — 75 kDa (red) and 25 kDa (green)
Contents
Approximately 0.3~0.8 mg/ml of each protein in the buffer (20 mM Tris-phosphate (pH 7.5), 2% SDS, 3.6 M urea, and 15% (v/v) glycerol).
Quality Control
Under suggested conditions, PM2510 ExcelBand™ Enhanced 3-color Regular Range Protein Marker resolves 10 major bands in 15% SDS-PAGE (Tris-Glycine buffer, MOPS, and MES buffer) and after Western blotting to nitrocellulose membrane.
Storage
4°C for 3 months
-20°C for long term storage
Specification
Cat. No. | PM2510 / PM2511 |
Series Name | ExcelBand™ |
Product Size | 250 μl x 2 / 250 μl x 10 |
MW Range | 10 – 180 kDa |
Band Number | 10 |
Band Color | Red/Green/Blue |
Markered Bands | 25, 75 kDa |
Manual
Manual_PM2510_ExcelBand™ Enhanced 3-color Regular Range Protein Marker (2025 ver. 3.4.0)
SDS
Migration patterns and approximate MWs (kDa)
Why are there contrasting results in molecular weights after using different brands of protein markers?
A. Different proteins even with similar molecular weights would exhibit apparent disparity from the resulting SDS PAGE due to the difference in the composition of the protein’s amino acids (e.g. gelatin). The reason for the disparity is due to the amino acids composition that affects the binding of the protein and SDS. Therefore, we can say that protein marker is a handy tool to estimate molecular weight, but there is no absolute molecular weight standard.
B. While running SDS-PAGE, protein mobility can be affected by the composition of the buffer used, gel percentage, the voltage used, running time, as well as if there is a pre-run.
C. Another recommendation for high molecular weight proteins is to prolong the running time to clarify the relative location of bands.
Protein marker Retention Period: Mentioned -20°C and over 2 years. Is it available for 30 months or 36 months? Have you tested this period?
Yes, we have tested our PM2700. The results showed that the PM2700 is stable at -20℃ for at least two years. It has also shown strong performance for more than 36 months under our careful storage. However, we must only suggest a 2 year retention period for the following reasons: There may be a variation in the environment in storage, and improper use may lead to accumulated damage to the proteins and therefore reduce its retention period.
How many times of freezing and thawing are available for protein markers? If it uses 5 μL per load, would the total usage quantity be 50 times x 2 (250 μL x 2 tube)?
Yes, 100 uses (5 μL each time) can be expected if freezing and thawing are conducted carefully and properly at the appropriate temperature. Before each use, make sure the protein marker is thoroughly thawed.
Do you have data comparison for protein molecular weight’s precision with other protein markers?
Yes. Usually, pre-stained marker is written on “estimated molecular weight” for caution. It is known that the analysis of protein size by an SDS-PAGE is only for “estimation” because of the intrinsic variation of amino acid composition in all proteins including stained and non-stained ones. For example, a protein which is highly hydrophilic might show a particular higher position in the SDS-PAGE analysis when compared to a hydrophobic one. We did compare the migration patterns of SMOBIO’s Protein Markers with other brands, and we concluded that it was difficult to define “precision” due to the reasons mentioned above. Therefore, in the product description, we suggest our users to calibrate the MW against their interested proteins. Although it is impossible to define "precision" for molecular weight of proteins in SDS-PAGE, we did compare the migration pattern of pre-stained markers with unstained protein marker (Invitrogen MARK12) for calibration. It is concluded that the estimated molecular weight of SMOBIO’s pre-stained marker shows a curve matching well with that of unstained native proteins (MARK12), representing a good estimation of the MW of each pre-stained protein in the SDS-PAGE analysis.
Will SMOBIO’s Protein Markers/Ladder be washed out during Western blotting process?
SMOBIO’s Protein Markers/Ladder will be only slightly washed out during Western blotting process. However, the excess of Tween-20 (more than 0.2%) in washing buffer will affect SMOBIO’s Protein Markers/Ladder on the transfer membrane.
Here are suggestions for Western blotting process:
1. Transfer SMOBIO’s Protein Markers/Ladder to membrane with transfer buffer containing 20% methanol to fix SMOBIO’s Protein Markers/Ladder on membrane.
2. Wash membrane with PBS or TBS containing less than 0.1% Tween-20.
Will SMOBIO’s Protein Markers/Ladder be affected by the stripping/deprobing process with the presence of β-Mercaptoethanol (β-ME)?
In normal circumstances, the presence of βME during the stripping/deprobing process will only slightly affect SMOBIO’s Protein Markers/Ladder. However, the presence of Tween-20 on PVDF membrane during the stripping/deprobing process has adverse effects on SMOBIO’s Protein Markers/Ladder.
Here are suggestions for Western stripping/deprobing process:
1. Wash the PVDF membrane in methanol for 5~10 minutes prior to the stripping/deprobing process to mitigate the adverse effect of Tween-20.2. Recommended stripping buffer (for 1 L):
15 g glycine,
1 g SDS,
10 mL Tween 20.
Dissolve in 800 mL distilled water.
Adjust pH to 2.2
Bring volume up to 1 L with distilled water
Jasmina Kubitschek, Vakil Takhaveev, Cécile Mingard, Martha I Rochlitz, Patricia B Reinert, Giulia Keller, Tom Kloter, Raúl Fernández Cereijo, Sabrina M Huber, Maureen McKeague, Shana J Sturla
Nucleic Acids Res. 2025 Jan 11;53(2):gkae1320. doi: 10.1093/nar/gkae1320.
PMCID: PMC11744188
Wang H, Wen J, Ablimit N, Deng K, Wang W, Jiang W.
Mar Drugs. 2024 Oct 2;22(10):453. doi: 10.3390/md22100453.
PMC11509462
Wang H, Wen J, Ablimit N, Deng K, Wang W, Jiang W.
Mar Drugs. 2024 Oct 2;22(10):453. doi: 10.3390/md22100453.
PMC11509462
Antigout effects and mechanisms of total flavonoids from prunus tomentosa
Yanan Jiang, Chengyi Zhang, Xinyue Zhang, Na Lan, Zihan Zhao, Yawei Xv, Qi Wang, Siwei Wang, Baifeng Chen, Xi Chen, Yilin Wang
Technol Health Care. 2024;32(S1):217-228. doi: 10.3233/THC-248019.
PMCID: PMC11191451
Pei-Chen Chen, Tzu-Pei Tsai, Yi-Chu Liao, Yu-Chieh Liao, Hung-Wei Cheng, Yi-Hsiu Weng, Chiao-Mei Lin, Cheng-Yuan Kao, Chih-Cheng Tai, Jhen-Wei Ruan
NPJ Biofilms Microbiomes. 2024; 10: 22. Published online 2024 Mar 13. doi: 10.1038/s41522-024-00495-8
PMCID: PMC10937957
Jun Heo, Chang Woo Kwon, Juno Lee, Haena Park, Hyunjong Yu, and Pahn-Shick Chang
Curr Res Food Sci. 2022; 5: 2081–2093. Published online 2022 Nov 2. doi: 10.1016/j.crfs.2022.10.033
PMCID: PMC9641177
Purification and Characterization of a Dark Red Skin Related Dimeric Polyphenol Oxidase from Huaniu Apples
Bin Liu, Xianfang Zhou, Haiyan Guan, Xuequn Pang and Zhaoqi Zhang, Foods. 2022 Jun 17;11(12):1790. doi: 10.3390/foods11121790.
PMCID: PMC9223062
Shuangying Chao, Yuhang Liu, Ning Ding, Yue Lin, Qian Wang, Junwen Tan, Wei Li, Yang Zheng, Xuejun Hu, Junming Li, Front Mol Biosci
. 2022 Mar 10;9:848829. doi: 10.3389/fmolb.2022.848829. eCollection 2022.
PMCID: PMC8960375
Shailima Rampogu, Seong Min Kim, Baji Shaik, Gihwan Lee, Ju Hyun Kim, Gon Sup Kim, Keun Woo Lee, Myeong Ok Kim, Front Oncol. 2021 Aug 18;11:712824. doi: 10.3389/fonc.2021.712824. eCollection 2021.
PMCID: PMC8416463
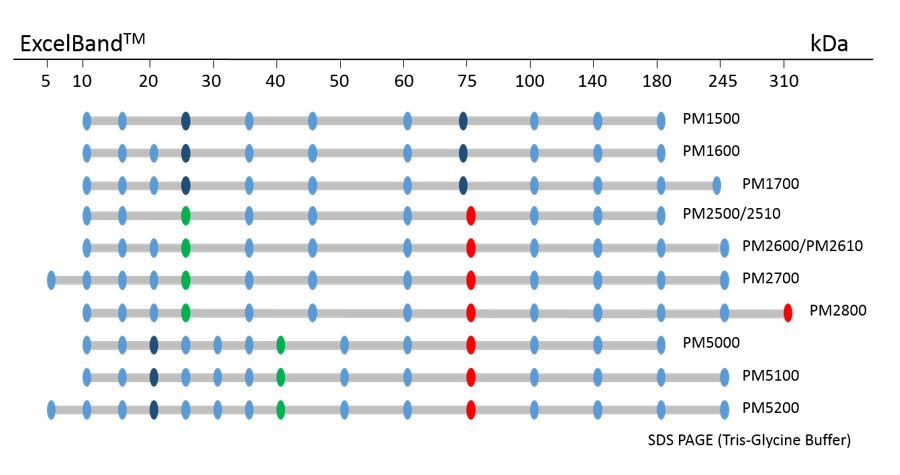
ExcelBand™ Protein Markers
Ready-to-use— premixed with a loading buffer for direct loading, no need to boil
Broad range— 310 kDa to 5 kDa
Pre-stained bands — for monitoring protein separation during electrophoresis and Western blotting transferring efficiency on membrane
Enhanced bands— for quick reference
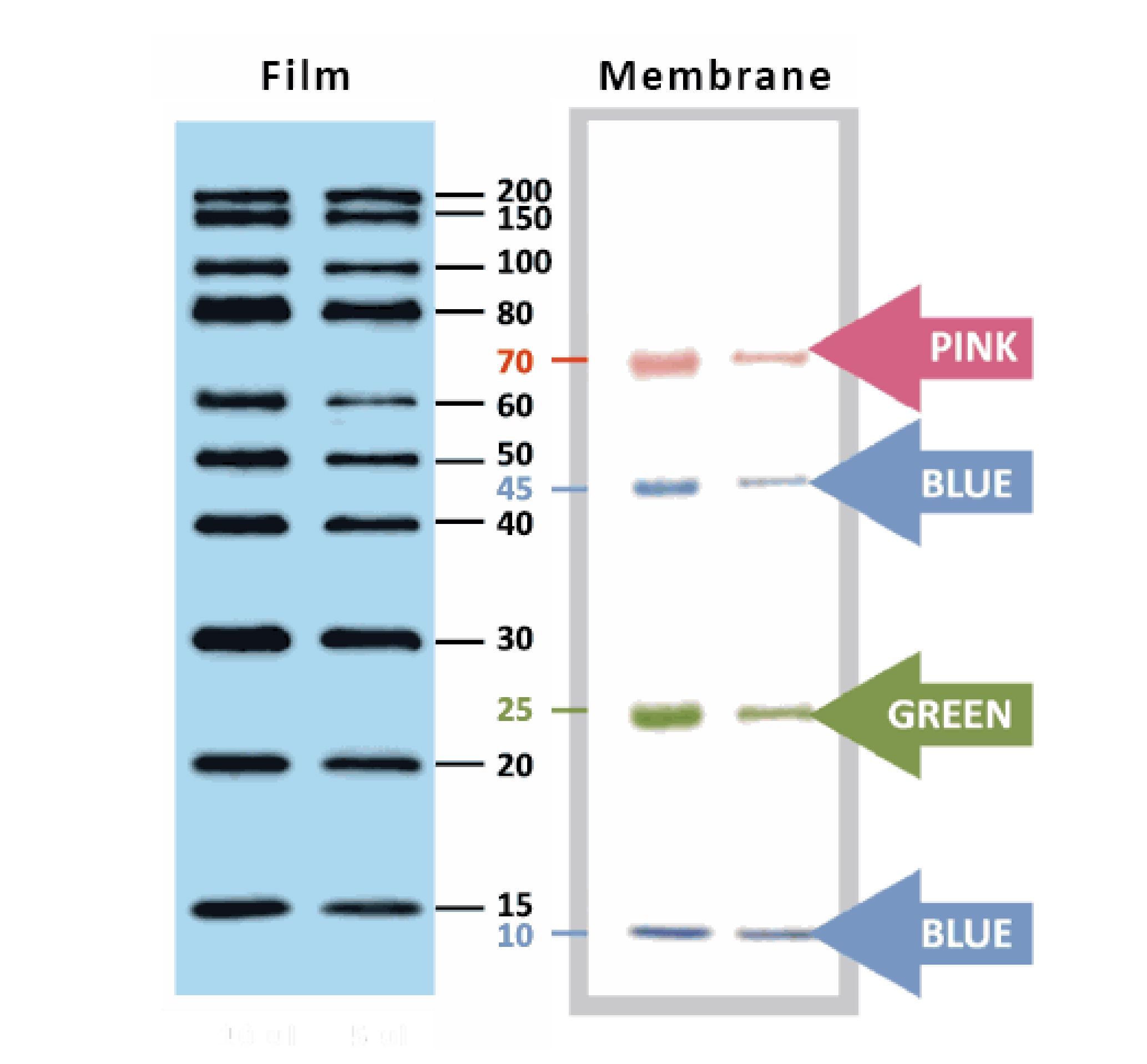
YesBlot™ Western Marker I
Ready-to-use — no need of mixing or heating before sample loading
Direct visualization — 10 IgG-binding proteins for direct visualization on Western blots
Pre-stained bands — 4 pre-stained proteins for monitoring protein separation during electrophoresis and Western blotting transferring efficiency on membrane
Wide range — 10 clear bands from 15 to 200 kDa for size estimation
Quick reference — two enhanced bands (30 and 80 kDa)
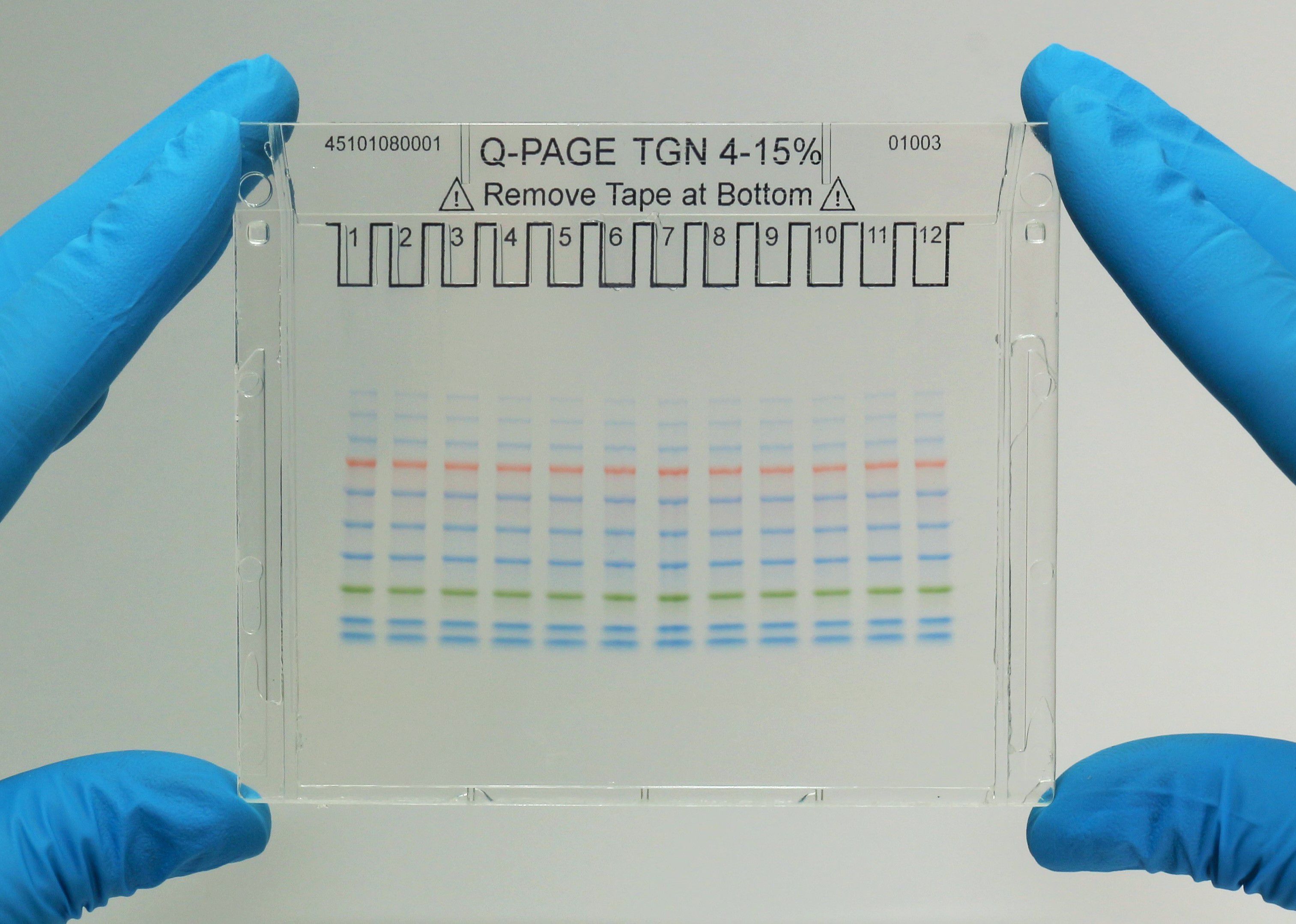
Q-PAGE™ Precast Gels
User-friendly gel cassette:
Numbered and framed wells for sample loading
Labeled warning sign and green tape as reminder
Enhanced gel performance:
Enhanced gel electrophoresis speed
Better band separation
Stable for shipping at ambient temperature
Easy compatibility:
Available as homogeneous and adjusted gradient gels for a wide range of protein separation.
Compatible with most popular protein electrophoresis systems

![[PM2510] ExcelBand™ Enhanced 3-color Regular Range Protein Marker (9-180 kDa), 250 μl x 2](/web/image/product.template/257/image?unique=5337757)
![[PM2510] ExcelBand™ Enhanced 3-color Regular Range Protein Marker (9-180 kDa), 250 μl x 2](/website/image/product.template/257/image/90x90)