Description
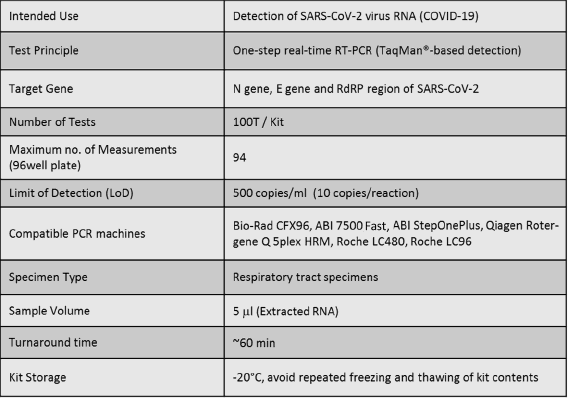
The IdPath™ COVID-19 Real-Time RT-PCR Kit is a real-time RT-PCR test intended for the detection of SARS-CoV-2 virus RNA which might be extracted from the respiratory tract specimens. The Kit provides reagents for multiplex real-time RT-PCR to detect SARS-CoV-2 by one-step reaction, specifically targeting the E (Envelope), RdRP (RNA-dependent RNA polymerase) and N (Nucleocapsid protein) gene for SARS-CoV-2 virus.
The Kit contains the RT enzyme mix and qPCR master mix for reverse transcription and real-time PCR of virus RNA. The COVID-19 Control (positive control) and ddWater (negative control) are used as indicators to avoid false negative/positive results across all experimental procedures. The Primers/probes Mix contains multiplex primers and TaqMan probes specific to the N, E/RdRP genes of SARS-CoV-2 and RNase P gene of human, detected by FAM, VIC and ROX channels, respectively.
For Research Use Only
Features
High Sensitivity:5×102 copies/ml (10 copies/rxn)
High Inclusivity : >99% of currently available complete virus genomes for SARS-CoV-2 including Omicron variant
High Accuracy : Clinical validation with 100% accuracy
High Stability : 37/25°C for 2 weeks ; 4°C for 24 weeks ; 10 times of freeze-thaw cycles
High Compatibility : Suitable for most laboratory qPCR machines
Operation Control : Including internal control for quality control of total process
Convenience : Multiplex (E/RdRP and N) detection by one-step reaction
Storage
Aliquot to avoid multiple freeze-thaw cycles
Protect fluorogenic probes from light
-20°C for 12 months
High Sensitivity:5×102 copies/ml (10 copies/rxn)
The confirmatory LOD for SARS-CoV-2 is 500 copies/ml for the IdPath™ COVID-19 Real-Time RT-PCR Kit when analyzed in Bio-Rad CFX96, ABI StepOnePlus, ABI 7500 Fast, Roche LC480, Roche LC96, as well as in Qiagen Rotor Gene Q qPCR instruments.
High Inclusivity :>99% of currently available complete virus genomes for SARS-CoV-2 including Omicron variant
Based upon BLAST analysis, the IdPath™ COVID-19 Real-Time RT-PCR Kit maps with >99.1% homology of known SARS-CoV-2 isolates in GenBank databases. Results confirmed the perfect matches to SARS-CoV-2 and inclusivity for 5 variants of concern are more than 99% in the database.
*Percentage of primer/probe matches with the sequences of SARS-CoV-2 (exhibiting ≤ 1 mismatch)
High Stability
The IdPath™ COVID-19 Real-Time RT-PCR Kit is stable at 37°C and 25°C for at least 2 weeks, and at 4°C for 24 weeks. The kit is also stable during 10 times of freeze-thaw cycles.
Clinical studies using contrived specimens
The data showed 100% agreement with the expected results in the RNA spiked specimens and 100% agreement with the expected results in the negative specimens. The results demonstrated that the IdPath™ COVID-19 Real-Time RT-PCR Kit has 100% sensitivity, 100% specificity and 100% accuracy.
Clinical validation using patient specimens
The results of 60 positive (Ct < 30: 30 samples and Ct 30-35: 30 samples) and 30 negative samples from the patient specimens were compared to the FDA-authorized comparator real-time RT-PCR assay (CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel). The data showed that the IdPath™ COVID-19 Real-Time RT-PCR Kit has 100% sensitivity, 100% specificity and 100% accuracy.
No Cross-Reactivity in Silico Analysis
An in silico analysis was performed to assess the potential of 47 organisms for interference with SARS-CoV-2 detection. The reference sequence (RefSeqs) in the NCBI Virus database or whole genome in the taxonomy database were used. No significant homology was observed between the SARS-CoV-2 primers/probes and other coronaviruses (except SARS-CoV) or human microflora.
No Cross-Reactivity in Wet Test
The cross-reactivity of the IdPath™ COVID-19 Real-Time RT-PCR Kit was also evaluated with the NATtrol™ Respiratory Verification Panel (Zeptometrix), which is comprised by 19 pathogens including those commonly found in respiratory tract. No cross-reactivity was observed between the SARS-CoV-2 primers/probes and 19 pathogens.
High Compatibility: Suitable for most laboratory qPCR machines
Applicable to Bio-Rad CFX96, ABI StepOnePlus, ABI 7500 Fast, Roche LC480, Roche LC96, as well as Qiagen Rotor Gene Q 5plex HRM qPCR instruments.
Product Information
Contents
Manual
Manual_IP2000_IdPath™ COVID-19 Real-Time RT-PCR Kit (2023 ver. 1.2.1)
Software Setting Guide
SDS
Flyer
IP2000 Operating Guide
Reagent Storage and Handling
Reagents, master mix, and RNA must be thawed and kept on a cold block at all times during preparation and use.
Do not push air into the assay mixtures and do not mix by violently pipetting up and down to avoid bubble generation.
Be sure not to introduce any foam or bubbles into the wells of 96-well plates when aliquoting reaction mix and nucleic acid.
As adding sample and control, immerse the tip below the surface of the liquid. Then press down the push button smoothly to the first stop.
Run on the qPCR machine immediately after mixture preparation is completed.
Recommended reaction mixture set up for qPCR
Component | 1 Reaction (Total 15 μl) | Volumes for N specimens (μl) |
Master Mix (B, Green) | 10 μl | 10 x (N+4) |
Enzyme Mix (E, Red) | 2 μl | 2 x (N+4) |
Primers/probes Mix (A, Amber) | 2 μl | 2 x (N+4) |
ddWater | 1 μl | 1 x (N+4) |
Sample | 5 μl | 5 x (N+4) |
Total volume | 20 μl | 20 x (N+4) |
Recommended qPCR Program
Fast program
Steps | Temp. | Time | Cycles |
Reverse transcription | 50°C | 10 min | 1 |
Enzyme activation | 95°C | 1 min | 1 |
Denaturation | 95°C | 3 sec | 40 |
Annealing/ Extension | 58°C | 30 sec |

![[IP2000] IdPath™ COVID-19 Real-Time RT-PCR Kit, 100 RXN](/web/image/product.template/477/image?unique=aff5c87)
![[IP2000] IdPath™ COVID-19 Real-Time RT-PCR Kit, 100 RXN](/web/image/product.image/297/image?unique=1b33a23)
![[IP2000] IdPath™ COVID-19 Real-Time RT-PCR Kit, 100 RXN](/website/image/product.template/477/image/90x90)
![[IP2000] IdPath™ COVID-19 Real-Time RT-PCR Kit, 100 RXN](/website/image/product.image/297/image/90x90)