IdPathTM COVID-19 Real-Time RT-PCR Kit
Introduction
A novel coronavirus (i.e., SARS-CoV-2) has now resulted in hundreds of millions of confirmed human infections globally. Cases of asymptomatic infection, mild illness, severe illness, and deaths have been reported. To curb the spread of COVID-19 (coronavirus disease 2019) pandemic, the world needs diagnostic systems capable of rapid and reliable detection of the novel coronavirus (SARS-CoV-2). The IdPathTM COVID-19 Real-Time RT-PCR Kit is developed and produced by SMOBIO, intended for the quantitative detection of nucleic acid from the SARS-CoV-2 in nasopharyngeal swab specimens collected from individuals who meet SARS-CoV-2 clinical criteria. This approach is based on the RT-PCR method, which uses two sets of gene-specific primers and corresponding fluorescent probes (TaqMan®) to detect three specific regions within the novel coronavirus (SARS-CoV-2) genes.
Methods
The SMOBIO IdPathTM COVID-19 Real-Time RT-PCR Kit includes the three primers and probe sets specific to the virus genomic regions of COVID-19 (N and E/RdRP gene). Purified nucleic acid is then reverse-transcribed into cDNA followed by PCR amplification and detection on Real-Time PCR instrument. Analytical performance of the IdPathTM COVID-19 Real-Time RT-PCR Kit was evaluated by determining Limit of Detection (LOD), assessing reactivity and cross-reactivity. In addition, a clinical evaluation study was performed for the IdPathTM COVID-19 Real-Time RT-PCR Kit.
Limit of Detection (LOD)
HIGH SENSITIVITY- 500 copies/mL for SARS-CoV-2
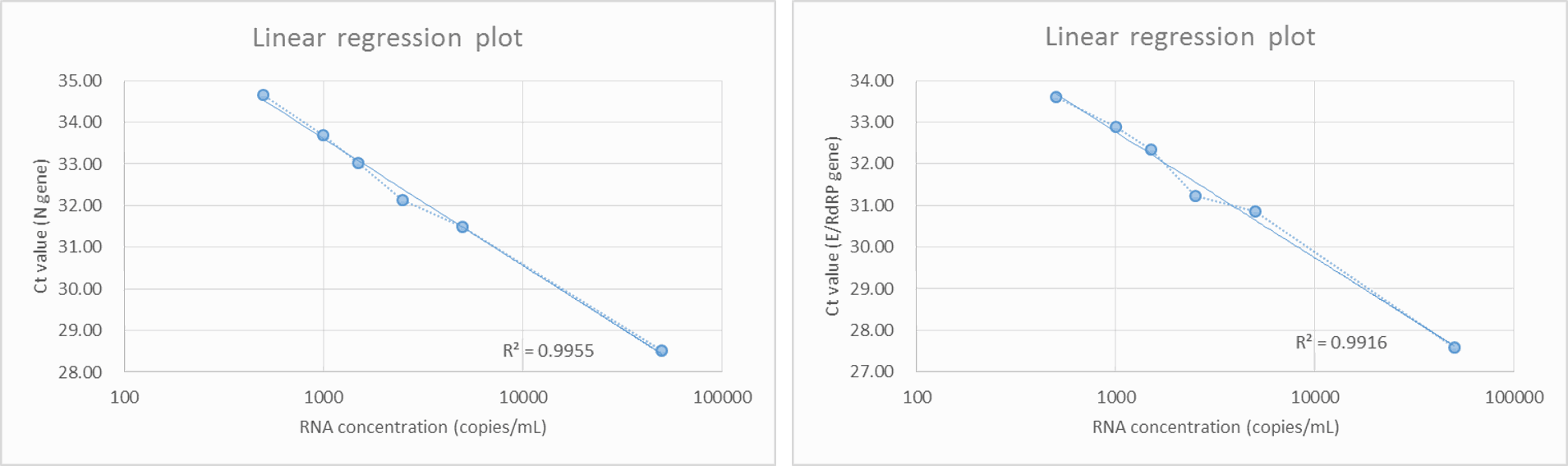
As shown in Figure 1a, preliminary LOD Range-Finding test was conducted by using individual negative swab specimens mixed with 250 to 50000 copies/mL of SARS-CoV-2 reference genomic RNA. All samples with RNA concentration over 250 copies/mL were positive for detection of N (FAM) and E/RdRP (VIC). The concentrations of SARS-CoV-2 reference genomic RNA and Ct values showed high linear correlation and the R2 values of both (N and E/RdRP genes) higher than 0.99 (Figure 1b). The confirmatory LOD for SARS-CoV-2 is 500 copies/mL for the IdPathTM COVID-19 Real-Time RT-PCR Kit when analyzed in Bio-Rad CFX96, ABI StepOnePlus, ABI 7500 Fast, Roche LC480, Roche LC96, as well as in Qiagen Rotor Gene Q qPCR instruments (See Fig. 2, 3a and 3b).
Figure 1a. Preliminary LoD Range-Finding Results (Bio-Rad CFX96)
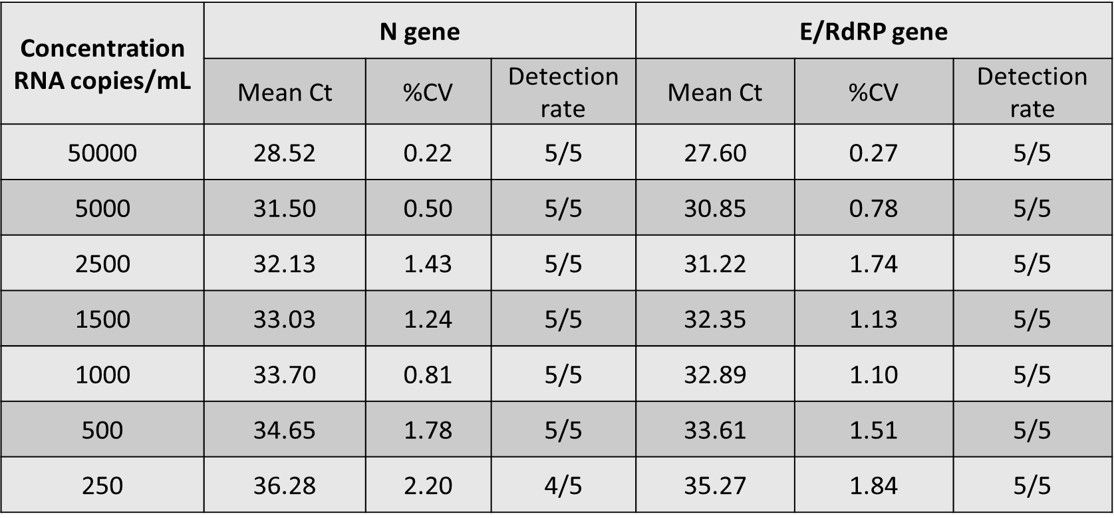
Figure 1b. Regression analysis of N and E/RdRP (Bio-Rad CFX96)
Figure 2. Confirmatory LOD Results (Bio-Rad CFX96)
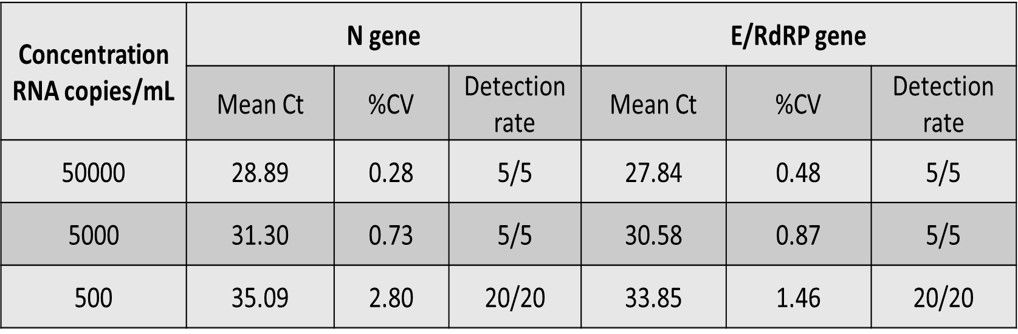
Figure 3a. LOD Summary on general qPCR machines
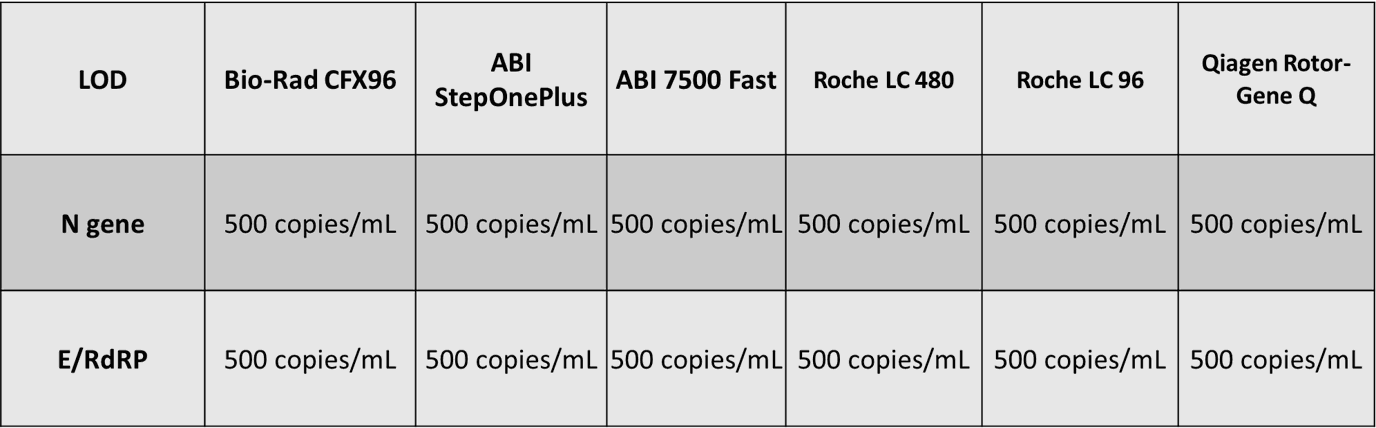
Figure 3b. Applicable qPCR instruments
UNIVERSALLY APPLICABLE -Suitable for most RT-PCR machines

Reactivity (Inclusivity): SARS-CoV-2 In Silico Analysis
HIGH SPECIFICITY- SARS-CoV-2 homology >99%
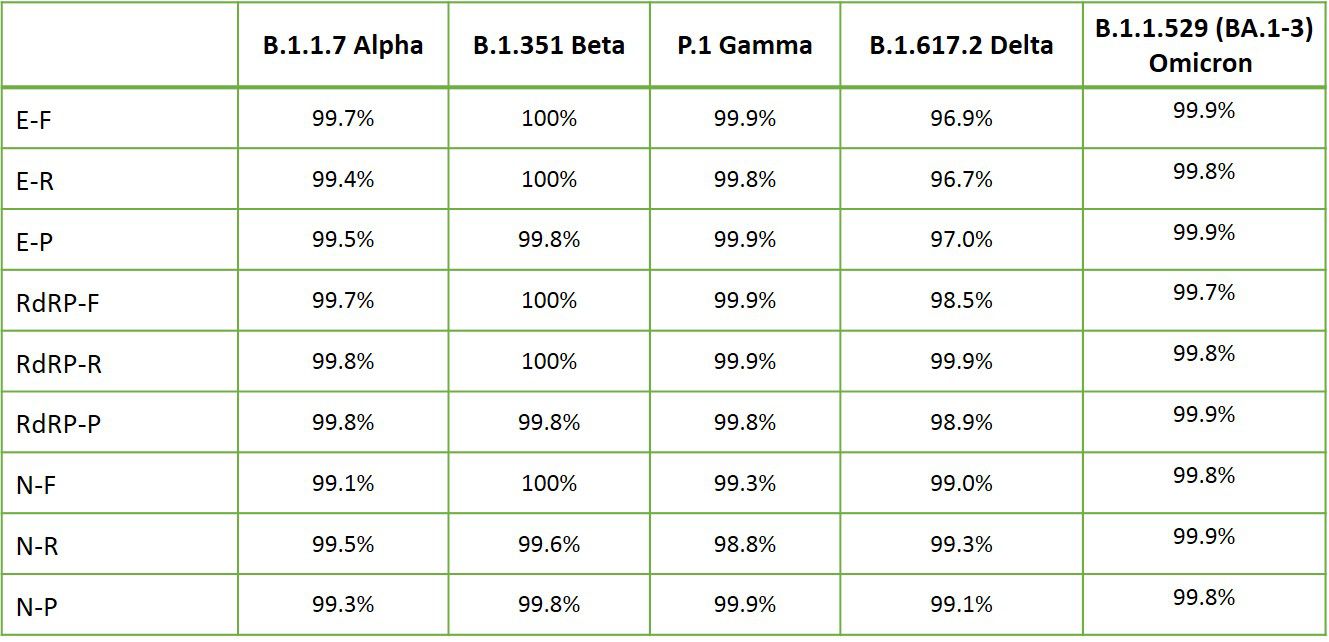
Based upon BLAST analysis, the IdPathTM COVID-19 Real-Time RT-PCR Kit maps with >99.1% homology of known SARS-CoV-2 isolates in GenBank databases. Results confirmed the perfect matches to SARS-CoV-2 and inclusivity for 5 variants of concern are more than 99% in the database (see Fig. 4~6).
Figure 4. In silico inclusivity analysis for SARS-CoV-2
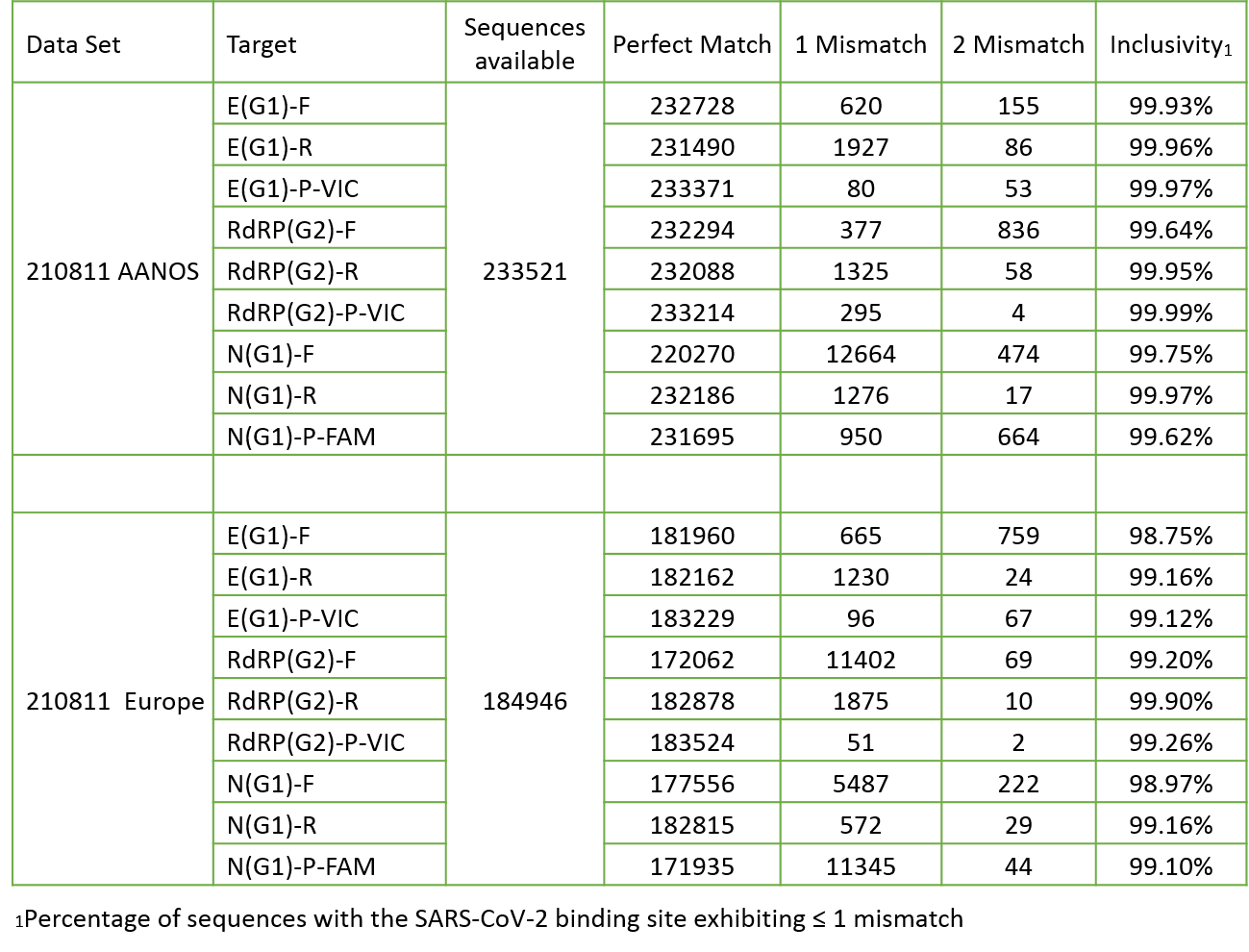
Figure 5. In silico inclusivity analysis for SARS-CoV-2 Omicron variant
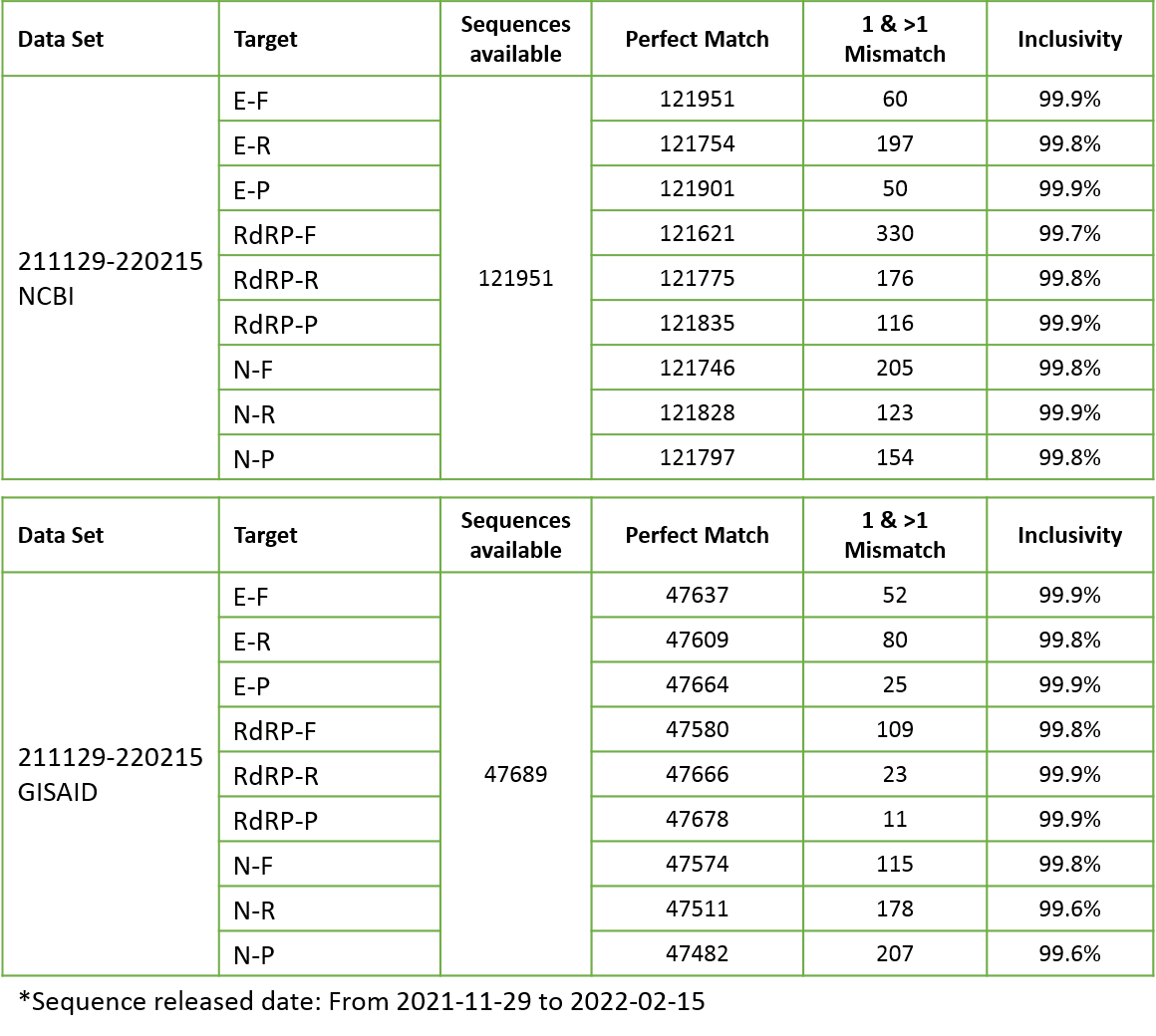
Figure 6. In silico inclusivity analysis for SARS-CoV-2 variants (WHO 5 variants of concern)
HIGH SPECIFICITY-VOC detection rate >99%
Analytical Specificity (Cross-Reactivity): SARS-CoV-2 In Silico Analysis
NO CROSS REACTIVITY DETECTED
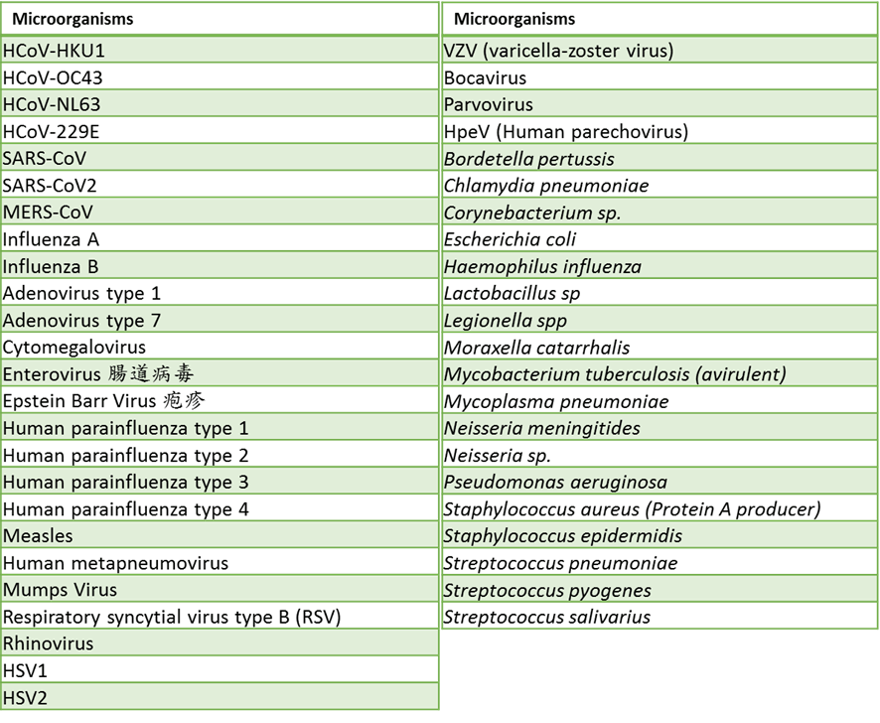
No significant homology was observed between the SARS-CoV-2 primers/probes and other coronaviruses (except SARS-CoV) or common human microflora (Fig. 7) that is predicted to lead to false results, indicating IdPath
TM
COVID-19 Real-Time RT-PCR Kit presents a good specificity.
Figure 7. Organisms and viruses evaluated for cross-reactivity by in silico analysis
Analytical Specificity (Cross-Reactivity): Wet Testing
NO CROSS REACTIVITY DETECTED
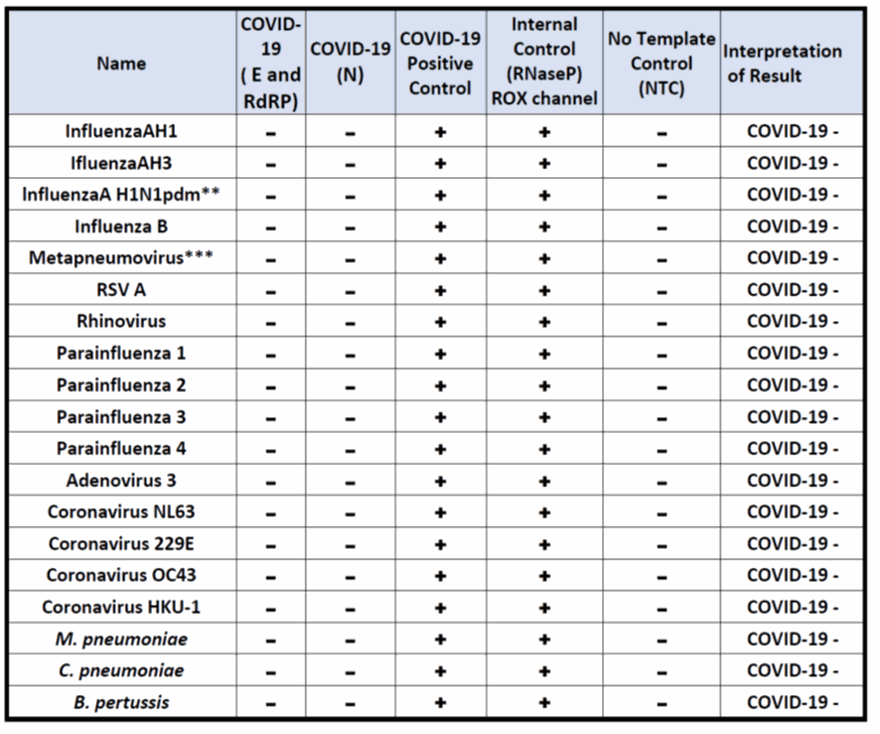
No cross-reactivity was observed between the SARS-CoV-2 primers/probes and 19 pathogens (as shown in Fig. 8).
Figure 8. Cross-reactivity testing results
Clinical Evaluation:
HIGH ACCURACY-100% of positive and negative agreement
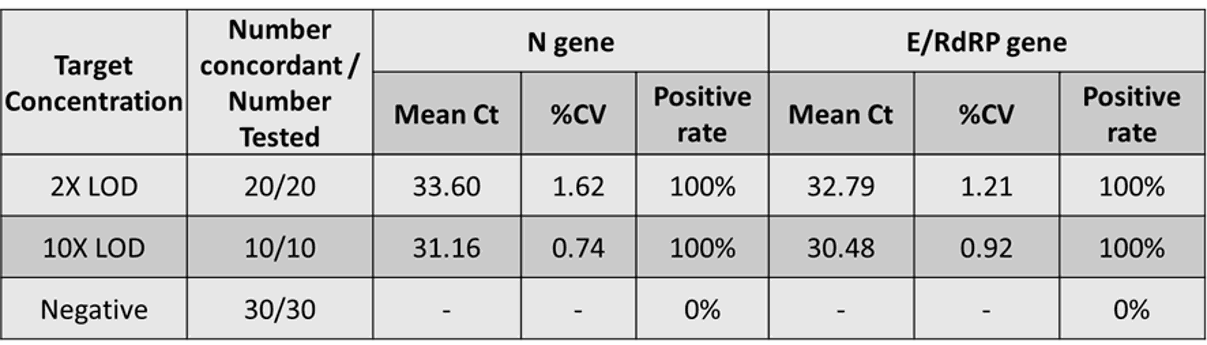
The results showed 100% agreement with the expected positive results in the RNA spiked specimens and 100% agreement with the expected negative results in the negative specimens (see Fig. 9).
Figure 9. Clinical performance evaluation (Bio-Rad CFX96)
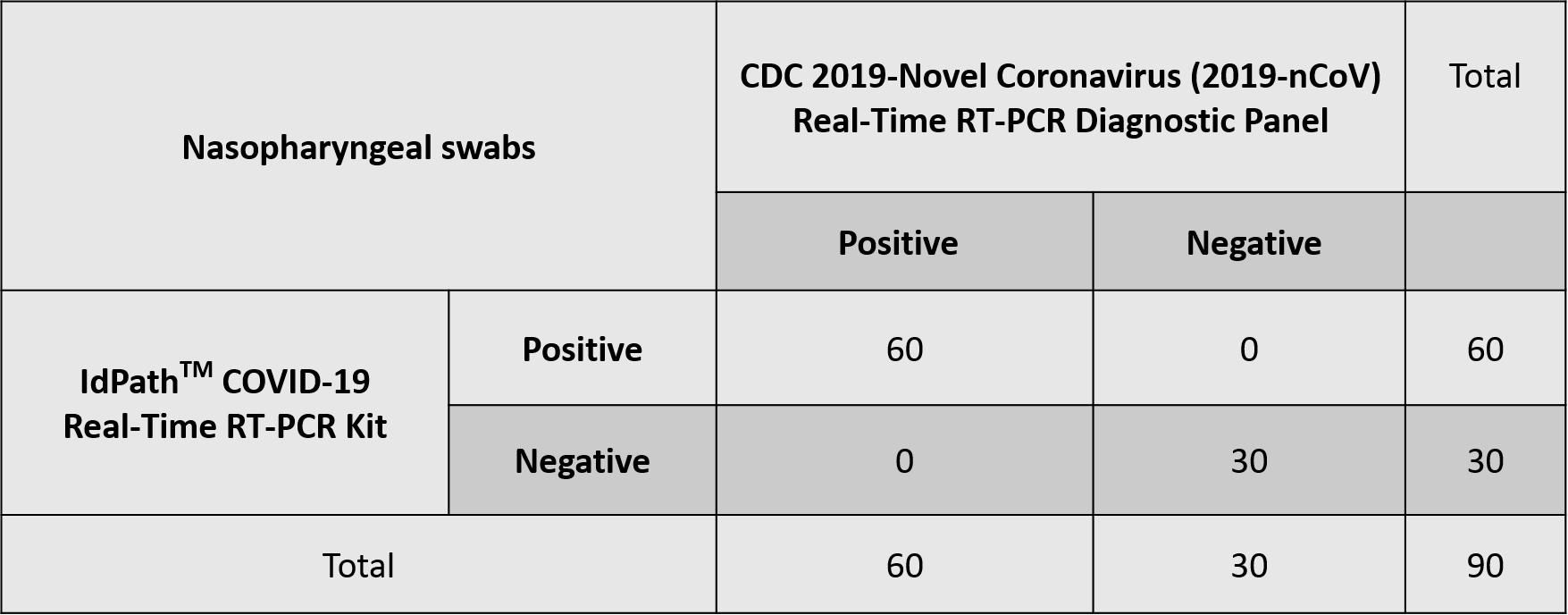
The test results for 60 positive and 30 negative clinical cases showed that the Positive Percent Agreement (PPA) is 100% and Negative Percent Agreement (NPA) is also 100%. IdPathTM COVID-19 Real-Time RT-PCR showed consistency for the detection of SARS-CoV-2 in comparison with the authorized NAAT Kit (see Fig. 10~11).
Figure 10. Specimen numbers used in the clinical performance evaluation (Unit: Case)
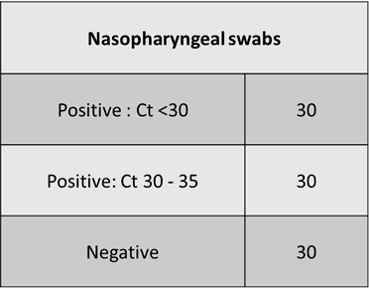
Figure 11. Clinical performance summary of
IdPath
TM
COVID -19
Real-Time RT-PCR Kit vs. an authorized NAAT Kit
Conclusion & Discussion:
IdPathTM COVID-19 Real-Time RT-PCR Kit specifically detects SARS-CoV-2 known to cause Covid-19. It will allow users to perform highly multiplex molecular detection in a simple work flow for Covid-19 identification.
● The Limit of Detections for SARS-CoV-2 is 500 copies/mL for general qPCR machines.
● The predicted inclusivity is over 99.1% homology of known SARS-CoV-2 isolates in GenBank databases and not impacted by the currently circulating 5 variants including Omicron.
● No cross-reactivity was observed with interfering pathogens panel test.
● Clinical performance tested with a set of 90 archived NPS samples gave overall 100% positive and 100% negative agreement, as compared to an authorized NAAT kit.