IdPathTM Flu/COVID-19 Real-Time RT-PCR Kit
Introduction
Symptoms of COVID-19 and influenza may look very similar, so it will be a challenge for clinicians to differentiate them if only based on signs and symptoms alone. To curb the spread of COVID-19 (coronavirus disease 2019) pandemic, the world needs diagnostic systems capable of rapid detection and differentiation of the novel coronavirus (SARS-CoV-2) and/or influenza A/B virus. The IdPathTM Flu/Covid-19 Real-Time RT-PCR Kit is developed and produced by SMOBIO, intended for the quantitative detection of nucleic acid from the SARS-CoV-2 and influenza A/B in nasopharyngeal swab specimens collected from individuals. This approach is based on the RT-PCR method, which uses three sets of gene-specific primers and corresponding fluorescent probes (TaqMan®) to detect three specific regions within the novel coronavirus (SARS-CoV-2), influenza A, and influenza B gene. This RT-PCR kit detects SARS-CoV-2 and influenza A/B ribonucleic acid (RNA) specifically.
Methods
The IdPathTM Flu/COVID-19 Real-Time RT-PCR Kit contains three primer/probe sets (InfA, InfB, and SC2) that target the RNA of influenza A, B and SARS-CoV-2 virus, respectively. The LOD of the kit was determined by spiking SARS-CoV-2, influenza A, and influenza B reference genome RNA into individual negative swab specimens. The analytical reactivity was evaluated by spiking 10 strains of influenza A and 5 strains of influenza B reference virus into individual negative swab specimens.
Limit of detection (LOD)
HIGH SENSITIVITY-
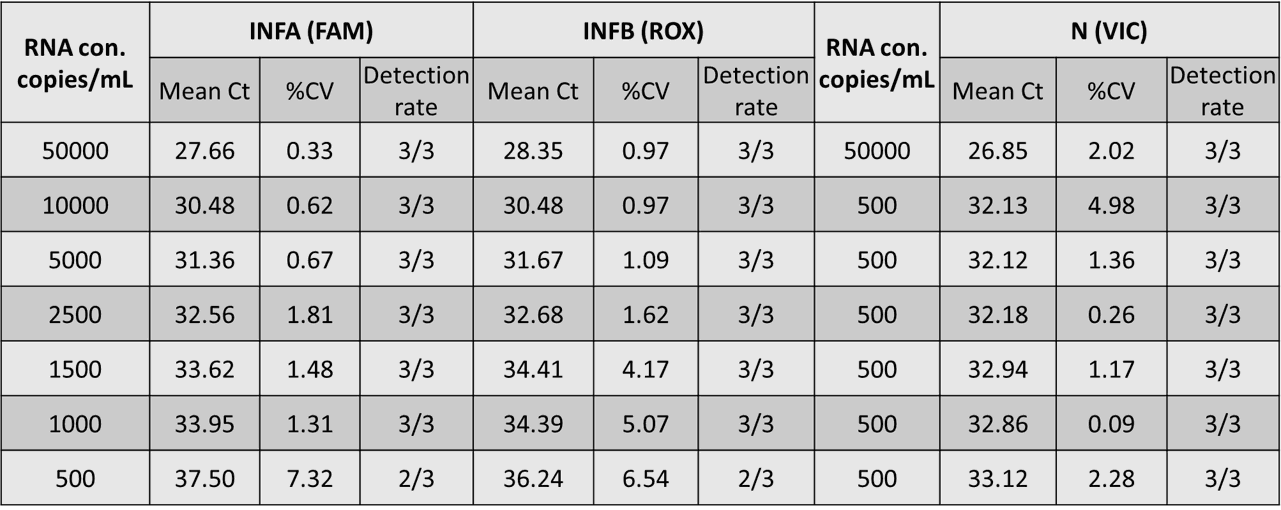
Influenza A : 1000 copies/mL
Influenza B : 1000 copies/mL
SARS-CoV-2 : 500 copies/mL
The preliminary LOD for influenza A, influenza B, and SARS-CoV-2 is respectively 1000, 1000 and 500 copy/mL with the IdPath
TM
Flu/COVID-19 Real-Time RT-PCR Kit (see Fig. 1).
Figure 1. Preliminary LOD Range-Finding Results (Bio-Rad CFX96)
Reactivity (Inclusivity): In Silico Analysis
HIGH SPECIFICITY-
Influenza A homology >96%
Influenza B homology >99%
SARS-CoV-2 homology >99%
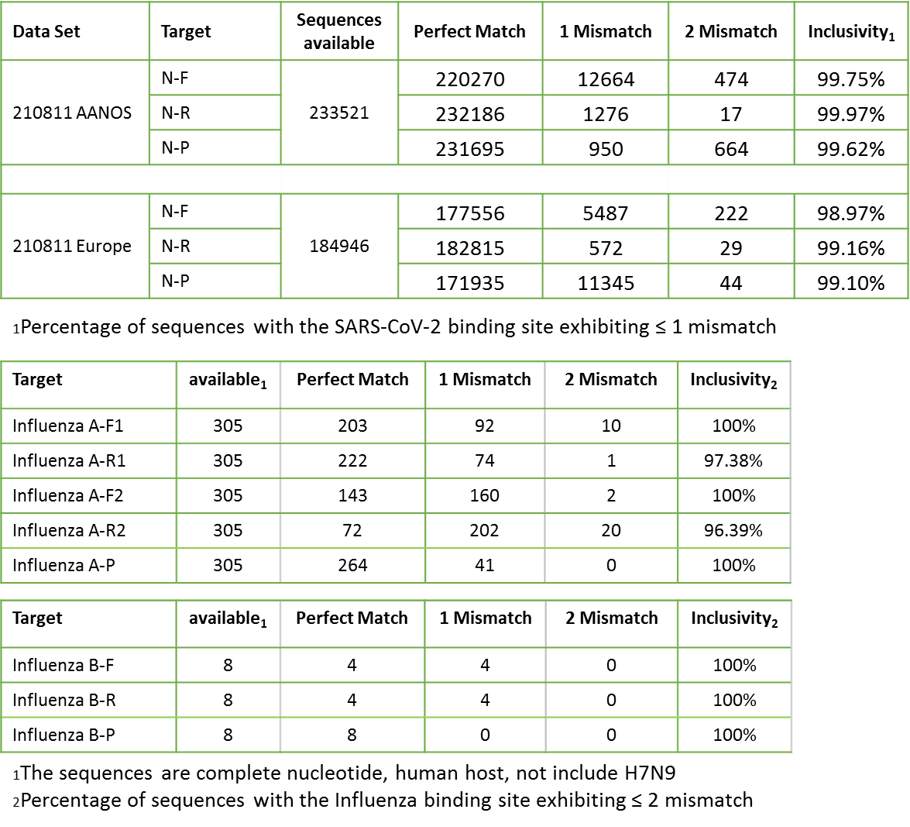
Based upon BLAST analysis, the IdPathTM Flu/Covid-19 Real-Time RT-PCR Kit maps respectively with over 99.4%, 96.39% and 100% homology of SARS-CoV-2, influenza A, and influenza B isolates in GenBank databases (Fig. 2).
Figure 2. In silico inclusivity analysis for SARS-CoV-2, influenza A, and influenza B.
Reactivity (Inclusivity): Wet test
All 10 strains of influenza A and 5 strains of influenza B reference virus were detected and accurately differentiated. IdPathTM Flu/COVID-19 Real-Time RT-PCR Kit has potential to reach high sensitivity and specificity for detection and differentiation of influenza A, influenza B, and SARS-CoV-2 virus (Fig. 3~4).
Figure 3. Inclusivity evaluation for influenza A viruses
Figure 4. Inclusivity evaluation for influenza B viruses.
Analytical Specificity (Cross-Reactivity): In Silico Analysis
NO CROSS REACTIVITY DETECTED
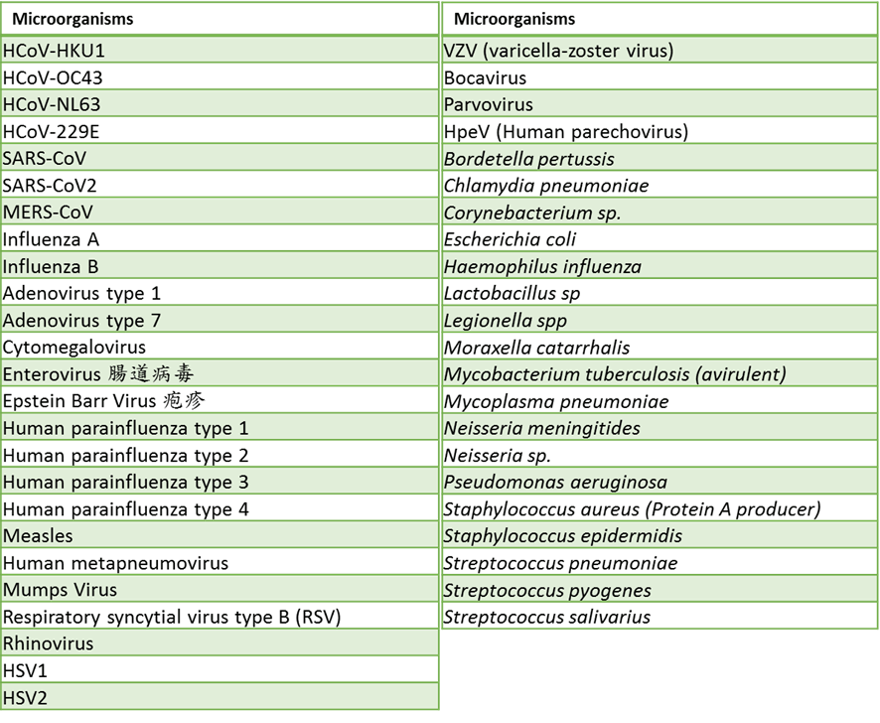
No significant homology was observed between the primers/probes (for SARS-CoV-2, influenza A, and influenza B) and other coronaviruses (except SARS-CoV) or human microflora that is predicted to lead to false results, indicating the IdPathTM Flu/COVID-19 Real-Time RT-PCR Kit presents a good specificity. (Fig. 5).
Figure 5. Organisms and viruses evaluated for cross-reactivity by in silico analysis
Conclusion & Discussion:
IdPathTM Flu/COVID-19 Real-Time RT-PCR Kit has potential to reach high sensitivity and specificity for detection and differentiation of influenza A, influenza B, and SARS-CoV-2 virus.
● The preliminary Limit of Detection (LOD) for influenza A, influenza B, and SARS-CoV-2 is 1000, 1000 and 500 copy/mL, respectively.
● The predicted inclusivity is over 99.4%, 96.39% and 100% homology of SARS-CoV-2, influenza A, and influenza B isolates, respectively, in GenBank databases.
● All 10 strains of influenza A and 5 strains of influenza B reference viruses were detected and accurately differentiated.
● No cross-reactivity was observed in silico analysis.
